您當前的位置:檢測資訊 > 法規標準
嘉峪檢測網 2025-05-12 19:38
近日,ECA 分析質量控制組(AQCG)制定了一份新文件:《實驗室數據管理指南——取樣與樣品管理》。 該文件強調了取樣作為一個潛在的誤差產生過程的至關重要性,并詳細說明了制定可靠取樣方案的要求,以將變異性降至最低并保持數據完整性。此外,該指南闡述了取樣過程中涉及的關鍵步驟,從統計取樣計劃到取樣方案設計,以及取樣記錄文件的編制等。

文件提及:取樣過程中,物料包裝密封容器的開啟、取樣及重新密封方式應能防止其內容物受到污染,同時防止對其他成分、藥品容器或密封件造成污染。
文件提及:如果需要從容器的頂部、中部和底部對組件進行取樣,則不得將此類分段取樣混合用于檢測。
文件提及:在物料質量被判定為合格之前,不得投入使用;在產品質量被判定為合格之前,不得進行銷售或供應。
文件還介紹了WHO 針對物料的三種簡化抽樣方案:
n方案(即根號N+1方案):WHO強烈警示了使用該方案的情況。對于那些需要對所接收的用于生產藥品的起始物料進行分析并決定放行或拒收的生產企業的質量控制實驗室而言,不建議使用n方案。
p 方案:當物料性質均一,且來自已認可的來源,同時主要目的是進行鑒別檢驗時,可以使用“p方案”
r 方案:當物料被懷疑性質不均一,以及/或者物料來自一個不太知名的來源時,可以使用 “r 方案”
該文件還提供了實際示例和說明性圖表,以幫助實驗室實施有效的取樣策略并確保符合法規要求。
文件翻譯如下:
LaboratoryDataManagementGuidance –Sampling
andSampleManagement–
實驗室數據管理指南——取樣與樣品管理
目錄
1 Introduction to AQCG & its Guidances
分析質量控制小組(AQCG)及其指南簡介
1.1 Guides and Guidances
指南和指導意見
2 Importance of Sampling and Sample Management
取樣和樣品管理的重要性
2.1 Sampling
取樣
2.2 Sample Management
樣品管理
3 Regulatory Requirements for Sampling and Sample Management
取樣及樣品管理的監管要求
3.1 USA cGMP (21 CFR §210ii & 21 CFR §211iii)
3.2 EU GMP
3.3 United States Pharmacopeia
美國藥典
3.4 WHO Guidance for sampling of pharmaceutical products and related materials
WHO 藥品及相關物料的取樣指導意見
3.5 FDA Guidance Investigating Out-of-Specification (OOS) Test Results for Pharmaceutical Production, May 2022
2022 年 5 月 FDA 藥品生產中OOS調查指南
3.6 GOOD Samples: Guidance on Obtaining Defensible Samples
良好取樣:獲取可靠樣品的指南
4 Theory of Sampling
抽樣理論
5 Nomenclature of sampling
抽樣的術語
6 The Sampling Process
抽樣過程
6.1 Statistical Sampling Plans
統計抽樣計劃
6.2 Acceptance Sampling Plans
驗收抽樣計劃
6.3 WHO guidelines; three reduced sampling plans for starting materials
WHO 針對起始物料的三種簡化抽樣方案
7 Sampling Procedure & Protocol
取樣程序&方案
7.1 In-process sampling
過程中取樣
8 Sampling Record
取樣記錄
9 Additional Guidance sources on sampling
關于取樣的其他指南
10 Sampling is only part of the Analysis and Testing Process
取樣只是分析和測試過程的一部分
11 References
參考文獻
1 Introduction to AQCG & its Guidances
分析質量控制小組(AQCG)及其指南簡介
The AQCG aims at providing a networking platform for Analytical Chemists and Scientists and to facilitate an active discussion of the latest regulatory requirements as well as identifying and addressing technical issues and challenges. In addition, it supports a harmonised approach through providing discussion/position papers and generic guidances via expert working groups to foster an effective and efficient communication between industry, competent authorities and the pharmacopoeias.
分析質量控制小組(AQCG)旨在為分析化學家和科學家提供一個交流平臺,推動對最新法規要求的積極討論,同時識別和解決技術問題與挑戰。此外,該小組通過專家工作組提供討論文件和立場文件以及通用指南,支持采用統一的方法,以促進行業、主管部門和藥典之間進行有效且高效的溝通。
1.1 Guides and Guidances
指南和指導意見
This guidance continues the sequence of these already published guidances which are available as pdf files for members of ECA.
本指南延續了此前已發布的一系列指導意見,這些指導意見以 PDF 文件的形式提供給歐洲化學工業協會(ECA)的會員。
• ECA _AQCG_ SOP 01_OOS_v2.0 August 2012
• ECA _AQCWG_ SOP 02_OOE OOT_v1.2_July 2023
• ECA _AQCG_ SOP 03_APLM_v1.0_July 2018
• ECA-AQCG-AIQSV-Guide-November 2023-v1.0
• ECA_Data Integrity Guide_v3.0 December 2022
2 Importance of Sampling and Sample Management
取樣和樣品管理的重要性
It is important to understand that sampling is always an error generating process and that although the reported result may be dependent upon the analytical method, it will always be dependent upon the sampling process.
取樣往往是一個會產生誤差的過程,而且盡管所報告的結果可能取決于分析方法,但它往往也取決于取樣過程。
The sampling process includes the specific activities undertaken to select the sample from a batch or lot and the management of the chain of custody of that sample throughout the lifecycle journey from sampling, through testing to retention and ultimate disposal. A high level process flow is shown in Figure 1.
取樣過程包括從批次中選取樣品所進行的特定活動,以及從取樣、測試到留樣和最終處置的整個生命周期中對該樣品的流轉的管理,其高層次的流程如圖 1 所示。

Figure 1 High level sampling and sample management process flow
圖 1 高層次的取樣及樣品管理流程
2.1 Sampling
取樣
Inconsistent sampling processes can lead to errors, resulting in data integrity issues and potentially incorrect decisions about pharmaceutical quality.
取樣過程不一致會導致誤差,進而引發數據完整性方面的問題,還可能使有關藥品質量的決策出現錯誤。
If the sample provenance cannot be assured such that the sample is representative of the overall batch or lot of material or product any testing is useless. It is essential that sampling process steps and flows are consistent and traceable
如果無法保證樣品的來源,使得樣品不能代表整批物料或產品,那么任何檢測都是徒勞的。至關重要的是,取樣的流程步驟必須一致且具有可追溯性。
2.2 Sample Management
樣品管理
Traceability is critical to demonstrate that the sample, as taken in accordance with the procedure is identified, protected and stored correctly prior to analysis. The sample record needs to contain all relevant data to confirm assurance that the sample tested has not been compromised prior to analysis in any way.
可追溯性至關重要,它能夠證明按照程序采集的樣品得到了識別、保護,并且在分析之前得到了正確的儲存。樣品記錄需要包含所有相關數據,以確保所測試的樣品在分析前未以任何方式受到影響。
In particular, the storage and transportation of samples activities should be strictly controlled.
特別是,樣品的儲存和運輸活動應當受到嚴格控制。
Ideally sampling should be performed in an area or booth designed for and dedicated to this purpose. However, if this is not possible sampling facilities should be designed to
理想情況下,取樣應在專門為此目的設計的區域或層流罩內進行。然而,如果無法做到這一點,取樣設施的設計應……
prevent contamination of the opened container, the materials and the operator;
防止已打開的容器、物料以及操作人員受到污染;
prevent cross-contamination by other materials, products and the environment;
防止受到其他物料、產品以及環境的交叉污染;
protect the individual who samples (sampler) during the sampling procedure.
在取樣過程中保護進行取樣人員
3 Regulatory Requirements for Sampling and Sample Management
取樣及樣品管理的監管要求
The requirements for sampling and sample management are scattered across multiple sources as illustrated in Figure 2. Details are provided in the subsequent sections 3.1 to 3.6 and their subsections.
取樣和樣品管理的要求分散在多個來源中,如圖2 所示。具體細節在后續的 3.1 至 3.6 節及其子節中給出。

Figure 2 Overview of sources of requirements for sampling and sample management
圖 2 取樣和樣品管理要求的來源
3.1 USA cGMP (21 CFR §210ii & 21 CFR §211iii)
3.1.1 21CFR§210.3 Definitions
Representative sample means a sample that consists of a number of units that are drawn based on rational criteria such as random sampling and intended to assure that the sample accurately portrays the material being sampled.
代表性樣品是指根據隨機取樣等合理標準抽取的由若干個單元組成的樣本,旨在確保樣本能夠準確地描繪被取樣的物料。
3.1.2 21 CFR §211.84 Testing and approval or rejection of components, drug product containers, and closures.
成分、藥品容器和密封件的檢測、批準或拒收
(a) Representative samples of each shipment of each lot shall be collected for testing or examination. The number of containers to be sampled, and the amount of material to be taken from each container, shall be based upon appropriate criteria such as statistical criteria for component variability, confidence levels, and degree of precision desired, the past quality history of the supplier, and the quantity needed for analysis and reserve where required by § 211.170.
每批貨物的每個批次都應采集代表性樣品進行測試或檢驗。待取樣的容器數量以及從每個容器中抽取的物料數量,應基于適當的標準來確定,如成分變異性的統計標準、置信水平、所需的精確程度、供應商過去的質量記錄,以及需要時,§211.170 所要求的分析和留樣的數量。
(b) Samples shall be collected in accordance with the following procedures:
樣品應按照以下程序采集:
1) The containers of components selected shall be cleaned when necessary in a manner to prevent introduction of contaminants into the component.
所選定的成分容器如有必要,應進行清潔,其清潔方式應能防止污染物進入成分中。
2) The containers shall be opened, sampled, and resealed in a manner designed to
prevent contamination of their contents and contamination of other components,
drug product containers, or closures.
容器的開啟、取樣及重新密封方式應能防止其內容物受到污染,同時防止對其他成分、藥品容器或密封件造成污染。
3) Sterile equipment and aseptic sampling techniques shall be used when necessary.
必要時,應使用無菌設備并采用無菌取樣技術。
4) If it is necessary to sample a component from the top, middle, and bottom of its container, such sample subdivisions shall not be composited for testing.
如果需要從容器的頂部、中部和底部對組件進行取樣,則不得將此類分段取樣混合用于檢測。
5) Sample containers shall be identified so that the following information can be determined: name of the material sampled, the lot number, the container from which the sample was taken, the date on which the sample was taken, and the name of the person who collected the sample.
樣品容器應予以標識,以便能夠確定以下信息:被取樣物料的名稱、批號、樣品所取自的容器、取樣日期以及取樣人員的姓名。
6) Containers from which samples have been taken shall be marked to show
that samples have been removed from them.
已從中取樣的容器應做好標識,以表明已從這些容器中取過樣。
3.1.3 21CFR§211.110 Sampling and testing of in-process materials and drugproducts.
中間物料和藥品的取樣與檢測
(a) To assure batch uniformity and integrity of drug products, written procedures shall be established and followed that describe the in-process controls, and tests, or examinations to be conducted on appropriate samples of in-process materials of each batch. Such control procedures shall be established to monitor the output and to validate the performance of those manufacturing processes that may be responsible for causing variability in the characteristics of in-process material and the drug product. Such control procedures shall include, but are not limited to, the following, where appropriate:
為確保藥品批次的均一性和完整性,應制定并遵循書面程序,描述對每批中間物料的適當樣品所進行的過程控制、測試或檢驗。此控制程序應建立以監控生產過程的產出,并驗證那些可能導致中間物料和藥品特性出現變異的生產工藝的性能。此控制程序應包含但不限于以下內容(如適用):
(1) Tablet or capsule weight variation;
片劑或膠囊的重量差異;
(2) Disintegration time;
崩解時限
(3) Adequacy of mixing to assure uniformity and homogeneity;
混合的充分性,以確保均勻性和同質性;
(4) Dissolution time and rate;
溶出時間和溶出速率;
(5) Clarity, completeness, or pH of solutions.
溶液的澄清度、完整性或pH。
(6) Bioburden testing.
生物負載測試。
3.1.4 21CFR§211.122 Materials examination and usage criteria.
物料檢驗及使用標準。
(a) There shall be written procedures describing in sufficient detail the receipt, identification, storage,handling, sampling, examination, and/or testing of labeling and packaging materials; such written procedures shall be followed. Labeling and packaging materials shall be representatively sampled, and examined or tested upon receipt and before use in packaging or labeling of a drug product.
應制定書面程序,詳細描述標簽和包裝材料的接收、識別、儲存、處理、取樣、檢查及/或測試;應遵循這些書面程序。標簽和包裝材料應進行有代表性的取樣,并在接收時以及用于藥品包裝或貼簽之前進行檢查或測試。
3.1.5 21CFR§211.134 Drug product inspection.
藥品檢驗
(a) Packaged and labeled products shall be examined during finishing operations to provide assurance that containers and packages in the lot have the correct label.
在成品包裝操作過程中,應對已包裝和貼標的產品進行檢查,以確保該批產品的容器和包裝上貼有正確的標簽。
(b) A representative sample of units shall be collected at the completion of finishing operations and shall be visually examined for correct labeling.
在成品包裝操作完成時,應采集具有代表性的產品樣品,并對其標簽是否正確進行目視檢查。
(c) Results of these examinations shall be recorded in the batch production or control records.
這些檢查的結果應記錄在批生產或控制記錄中。
3.1.6 21CFR§211.160 General requirements.
一般要求
(a) The establishment of any specifications, standards, sampling plans, test procedures, or other laboratory control mechanisms required by this subpart, including any change in such specifications, standards, sampling plans, test procedures, or other laboratory control mechanisms, shall be drafted by the appropriate organizational unit and reviewed and approved by the quality control unit. The requirements in this subpart shall be followed and shall be documented at the time of performance. Any deviation from the written specifications,standards, sampling plans, test procedures, or other laboratory control mechanisms shall be recorded and justified.
本部分所要求的任何規格、標準、取樣計劃、檢驗程序或其他實驗室控制機制的制定,包括對這些規格、標準、取樣計劃、檢驗程序或其他實驗室控制機制的任何變更,均應由適當的組織單位起草,并由質量控制部門進行審核和批準。應遵循本部分的要求,并在執行時做好記錄。任何偏離書面規格、標準、取樣計劃、檢驗程序或其他實驗室控制機制的情況都應予以記錄并說明理由。
(b) Laboratory controls shall include the establishment of scientifically sound and appropriate specifications, standards, sampling plans, and test procedures designed to assure that components, drug product containers, closures, in-process materials, labeling, and drug products conform to appropriate standards of identity, strength, quality, and purity. Laboratory controls shall include:
實驗室控制應包括制定科學合理且適當的規格、標準、取樣計劃和檢驗程序,以確保組分、藥品容器、密封件、中間物料、標簽和藥品符合有關鑒別、含量、質量和純度的適當標準。實驗室控制應包括:
(1) Determination of conformity to applicable written specifications for the acceptance of each lot within each shipment of components, drug product containers, closures, and labeling used in the manufacture, processing, packing, or holding of drug products. The specifications shall include a description of the sampling and testing procedures used. Samples shall be representative and adequately identified. Such procedures shall also require appropriate retesting of any component, drug product container, or closure that is subject to deterioration.
確定用于藥品生產、加工、包裝或儲存的每一批組分、藥品容器、密封件和標簽是否符合適用的書面標準以決定其是否可接受。這些標準應包括所使用的取樣和檢驗程序的說明。樣品應具有代表性并得到充分標識。這些程序還應要求對任何可能變質的組分、藥品容器或密封件進行適當的重新檢驗。
(2) Determination of conformance to written specifications and a description of sampling and testing procedures for in-process materials. Such samples shall be representative and properly identified.
確定中間物料是否符合書面標準,并對取樣和測試程序進行說明。此類樣品應具有代表性,并進行妥善標識。
(3) Determination of conformance to written descriptions of sampling procedures and appropriate specifications for drug products. Such samples shall be representative and properly identified.
確定藥品是否符合取樣程序的書面描述以及相應的標準要求。此類樣品應具有代表性,并進行妥善標識
3.1.7 21CFR§211.166 Stability testing
穩定性測試
The written program shall be followed and shall include:
應遵循書面程序,且該程序應包括:
(1) Sample size and test intervals based on statistical criteria for each attribute examined to assure valid estimates of stability;
基于統計學標準確定的樣品量和測試間隔,用于考察每一屬性,以確保對穩定性進行有效評估;
(2) Storage conditions for samples retained for testing;
用于測試而留存的樣品的儲存條件
3.1.8 21CFR§211.170 Reserve samples.
留樣
(a) An appropriately identified reserve sample that is representative of each lot in each shipment of each active ingredient shall be retained. The reserve sample consists of at least twice the quantity necessary for all tests required to determine whether the active ingredient meets its established specifications, except for sterility and pyrogen testing.
應當留存經適當標識、能代表每批活性成分發貨的留樣。除了無菌性和熱原性測試之外,留樣的數量至少應為確定該活性成分是否符合既定標準所需進行的所有測試所需數量的兩倍。
3.1.9 21CFR§211.186 Master production and control records
主生產及控制記錄
(9) Complete manufacturing and control instructions, sampling and testing procedures, specifications, special notations, and precautions to be followed.
完成需遵循的制造與控制說明、取樣及測試程序、規格、特殊注釋以及預防措施。
3.1.10 21CFR§211.194 Laboratory records
實驗室記錄
(a) Laboratory records shall include complete data derived from all tests necessary to assure compliance with established specifications and standards, including examinations and assays, as follows:
實驗室記錄應包括為確保符合既定規格和標準而進行的所有必要測試所產生的完整數據,其中包括檢驗和分析,具體如下:
(1) A description of the sample received for testing with identification of source (that is, location from where sample was obtained), quantity, lot number or other distinctive code, date sample was taken, and date sample was received for testing.
對接收用于測試的樣品的描述,包括樣品來源的標識(即取樣地點)、數量、批號或其他獨特的編碼、取樣日期以及接收樣品用于測試的日期。
(2) A statement of the weight or measure of sample used for each test, where
appropriate.
適當時,說明用于每項測試的樣品重量或度量情況。
3.2 EU GMPv
3.2.1 Part1Chapter4 Documentation
文件
Sampling
取樣
4.25 There should be written procedures for sampling, which include the methods and equipment to be used, the amounts to be taken and any precautions to be observed to avoid contamination of the material or any deterioration in its quality.
應當有關于取樣的書面程序,其中包括所使用的方法和設備、取樣數量,以及為避免物料受到污染或質量下降而需遵守的任何預防措施。
3.2.2 Part1 Chapter6 Quality Control
質量控制
Quality Control is concerned with sampling, specifications and testing as well as the organisation, do cumentation and release procedures which ensure that the necessary and relevant tests are carried out, and that materials are not released for use, nor products released for sale or supply, until their quality has been judged satisfactory.
質量控制涉及取樣、標準要求、測試,以及組織安排、文件記錄和放行程序。這些方面確保進行必要且相關的測試,并且在物料質量被判定為合格之前,不得投入使用;在產品質量被判定為合格之前,不得進行銷售或供應。
6.11 The sample taking should be done and recorded in accordance with approved written procedures that describe:
樣品的采集應按照經批準的書面程序進行并記錄,這些程序應描述:
i. The method of sampling
取樣的方法
ii. The equipment to be used
所使用的設備
iii.The amount of the sample to be taken
所取樣品的數量
iv. Instructions for any required sub-division of the sample
有關對樣品進行任何必要細分的說明
v. The type and condition of the sample container to be used
待使用的樣品容器的類型和狀況
vi. The identification of containers sampled
已取樣容器的標識
vii. Any special precautions to be observed, especially with regard to the sampling of sterile or noxious materials
需遵守的任何特殊預防措施,尤其涉及無菌或有害物料的取樣方面
viii. The storage conditions
儲存條件
ix. Instructions for the cleaning and storage of sampling equipment.
取樣設備的清潔和儲存說明。
6.12 Samples should be representative of the batch of materials or products from which they are taken. Other samples may also be taken to monitor the most stressed part of a process (e.g.beginning or end of a process). The sampling plan used should be appropriately justified and based on a risk management approach.
樣品應能代表從中抽取的那批物料或產品。也可抽取其他樣品來監控工藝中承受最大壓力(或最關鍵)的部分(例如,工藝的開始階段或結束階段)。所采用的取樣計劃應充分論證,并應基于風險管理方法制定。
6.13 Sample containers should bear a label indicating the contents, with the batch number, the date of sampling and the containers from which samples have been drawn. They should be managed in a manner to minimize the risk of mix-up and to protect the samples from adverse storage conditions.
樣品容器應貼有標簽,標明容器內的物質、批號、取樣日期以及樣品的來源容器。樣品容器的管理方式應能最大程度降低混淆的風險,并保護樣品免受不良儲存條件的影響。
3.2.3 Annex8 SAMPLING OF STARTING AND PACKAGING MATERIALS
附錄 8 起始物料和包裝材料的取樣
Personnel who take samples should receive initial and on-going regular training in the disciplines relevant to correct sampling.
取樣人員應接受與正確取樣相關學科的初始培訓和持續定期培訓。
This training should include:
這種培訓應包括:
• sampling plans
取樣計劃
• written sampling procedures
書面取樣程序
• the techniques and equipment for sampling
取樣技術和設備
• the risks of cross-contamination
交叉污染的風險
• the precautions to be taken with regard to unstable and/or sterile substances
對于不穩定和/或無菌物質需采取的預防措施
• the importance of considering the visual appearance of materials, containers and labels
考慮物料、容器和標簽的外觀情況的重要性。
• the importance of recording any unexpected or unusual circumstances
記錄任何意外或異常情況的重要性。
Starting materials
起始物料
The identity of a complete batch of starting materials can normally only be ensured if individual samples are taken from all the containers and an identity test performed on each sample.
通常只有從所有容器中都抽取個別樣品,并對每個樣品都進行鑒別測試,才能確保整批起始物料的身份(即確認物料的正確屬性)。
The quality of a batch of starting materials may be assessed by taking and testing a representative sample. The samples taken for identity testing could be used for this purpose.The number of samples taken for the preparation of a representative sample should be determined statistically and specified in a sampling plan. The number of individual samples which may be blended to form a composite sample should also be defined, taking into account the nature of the material, knowledge of the supplier and the homogeneity of the composite sample.
一批起始物料的質量可以通過抽取具有代表性的樣品并進行測試來評估。用于鑒別測試的樣品可用于此目的。為制備代表性樣品而抽取的樣品數量應通過統計方法確定,并在取樣計劃中予以明確規定。考慮到物料的性質、對供應商的了解以及混合樣品的均勻性,還應確定可混合以形成混合樣品的單個樣品的數量。
Packaging materials
包裝材料
The sampling plan for packaging materials should take account of at least the following;
包裝材料的取樣計劃至少應考慮以下因素:
• the quantity received
接收的數量
• the quality required
所需的質量
• the nature of the material (e.g. primary packaging materials and/or printed packaging materials)
材料的性質(例如,內包裝材料和/或印刷包裝材料)
• the production methods, and what is known of the Quality Assurance system of the packaging materials manufacturer based on audits.
生產方法,以及根據審計所了解的包裝材料制造商的質量保證體系。
The number of samples taken should be determined statistically and specified in a sampling plan.
抽取的樣本數量應通過統計方法來確定,并在取樣計劃中明確規定。
3.2.4Annex19 Reference and Retention Samples(also Annex13 IMPs)
附錄19 對照品和留樣(也參照附錄13 IMP)
This Annex to the Guide to Good Manufacturing Practice for Medicinal Products (“the GMP Guide”)gives guidance on the taking and holding of reference samples of starting materials, packaging materials or finished products and retention samples of finished products.
本《藥品生產質量管理規范》(“《GMP 規范》”)附錄就起始物料、包裝材料或成品的對照樣品的抽取和保存以及成品留樣提供指導。
2.1 Samples are retained to fulfil two purposes; firstly to provide a sample for analytical testing and secondly to provide a specimen of the fully finished product.
留樣有兩個目的:第一是為分析檢測提供樣品,第二是留存完整成品的樣本。
Samples may therefore fall into two categories
因此,樣品可分為兩類。
i. Reference sample: a sample of a batch of starting material, packaging material or finished product which is stored for the purpose of being analysed should the need arise during the shelf life of the batch concerned. Where stability permits, reference samples from critical intermediate stages (e.g. those requiring analytical testing and release) or intermediates, that are transported outside of the manufacturer’s control, should be kept.
參考樣品:一批起始物料、包裝材料或成品的樣品,保存該樣品是為了在有關批次的保質期內,在有需要時對其進行分析。在穩定性允許的情況下,應保留來自關鍵中間階段(例如,那些需要進行分析測試和放行的階段)的參考樣品,或者是需要運輸到制造商控制范圍之外的中間體的參考樣品。
ii. Retention sample: a sample of a fully packaged unit from a batch of finished product. It is stored for identification purposes. For example, presentation, packaging, labelling,patient information leaflet, batch number, expiry date should the need arise during the shelf life of the batch concerned.
留樣:從一批成品中取出的一個完整包裝單元的樣品。留樣是為了進行識別。例如,在有關批次的保質期內,當有需要時,留樣可用于識別產品的展示形式、包裝、標簽、患者信息單、批號、有效期等方面。
2.3 The reference and/or retention samples serve as a record of the batch of finished product or starting material and can be assessed in the event of, for example, a dosage form quality complaint, a query relating to compliance with the marketing authorisation, a labelling/packaging query or a pharmacovigilance report
參考樣品和/或留樣可作為成品批次或起始物料批次的記錄,并且在出現例如劑型質量投訴、與上市許可合規性相關的疑問、標簽/包裝方面的疑問或藥物警戒報告等情況時可用于評估。
2.4 Records of traceability of samples should be maintained and be available for review by competent authorities.
應保留樣品的可追溯性記錄,且這些記錄應可供主管部門查閱。
3.2.5 Part2;6.60 Laboratory Contro lRecords
實驗室控制記錄
A description of samples received for testing, including the material name or source, batch number or other distinctive code, date sample was taken, and, where appropriate, the quantity and date the sample was received for testing; -A statement of or reference to each test method used; -A statement of the weight or measure of sample used for each test as described by the method; data on or cross-reference to the preparation and testing of reference standards, reagents and standard solutions.
對收到用于檢測的樣品的描述,包括物料名稱或來源、批號或其他獨特編碼、樣品采集日期,以及在適當情況下,收到用于檢測的樣品的數量和日期;對所使用的每種檢測方法的說明或引用;對按照該方法所使用的樣品重量或度量的說明;關于參比標準品、試劑和標準溶液的制備和檢測的數據,或對這些數據的相互參照。
3.2.6 Part2;7.33-7.35 Sampling and Testing of Incoming Production Materials
進廠生產物料的取樣與檢驗
7.33 Samples should be representative of the batch of material from which they are taken.Sampling methods should specify the number of containers to be sampled, which part of the container to sample, and the amount of material to be taken from each container. The number of containers to sample and the sample size should be based upon a sampling plan that takes into consideration the criticality of the material, material variability, past quality history of the supplier, and the quantity needed for analysis.
樣品應能代表從中抽取樣品的那批物料。取樣方法應規定要取樣的容器數量、從容器的哪個部位取樣,以及從每個容器中抽取的物料數量。要取樣的容器數量和樣品量應基于一個取樣計劃,該計劃需考慮物料的關鍵程度、物料的變異性、供應商過去的質量記錄,以及分析所需的數量。
7.34 Sampling should be conducted at defined locations and by procedures designed to prevent contamination of the material sampled and contamination of other materials.
取樣應在規定的地點進行,并且要按照既定的程序操作,以防止被取樣的物料受到污染,同時也要避免對其他物料造成污染。
7.35 Containers from which samples are withdrawn should be opened carefully and subsequently reclosed. They should be marked to indicate that a sample has been taken.
從其抽取樣品的容器應小心開啟,隨后重新封閉。這些容器應做好標記,以表明已進行取樣操作。
3.2.7 Part2;7.8.35 In-process Sampling and Controls
過程中取樣和控制
In-process sampling should be conducted using procedures designed to prevent contamination of the sampled material and other intermediates or APIs. Procedures should be established to ensure the integrity of samples after collection.
過程中取樣應采用旨在防止被取樣物料以及其他中間體或原料藥受到污染的程序進行。應制定程序以確保樣品采集后的完整性。
3.2.8 Part2;11.52 Stability Monitoring of APIs
原料藥的穩定性監測
Stability samples should be stored in containers that simulate the market container. For example, if the API is marketed in bags within fiber drums, stability samples can be packaged in bags of the same material and in smaller- scale drums of similar or identical material composition to the market drums.
穩定性樣品應儲存在模擬上市包裝的容器中。例如,如果原料藥以裝在纖維桶內的袋子形式上市,穩定性樣品可以包裝在由相同材料制成的袋子中,并放在材料組成與上市包裝的桶相似或相同的小尺寸桶內。
3.2.9 Part2;11.7 Reserve/Retention Samples
備用/留樣
11.70 The packaging and holding of reserve samples is for the purpose of potential future evaluation of the quality of batches of API and not for future stability testing purposes.
備用樣品的包裝和保存是為了將來有可能對原料藥批次的質量進行評估,而并非用于將來的穩定性測試。
3.2.10 Part2;19.8 Laboratory Controls
實驗室控制
19.81 A system for retaining reserve samples of all batches should be in place. This system should ensure that a sufficient quantity of each reserve sample is retained for an appropriate length of time after approval, termination, or discontinuation of an application.
應建立一個保留所有批次備用樣品的系統。該系統應確保在申請獲批、終止或停止后,每個備用樣品都能保留足夠的數量和適當的時間。
3.3 United States Pharmacopeia
美國藥典
3.3.1 General Notices
總則
Sampling is not addressed in detail in the USP as the monographs are standards not specifications and apply to any tested sample (official article).
《美國藥典》中未詳細闡述取樣問題,因為各專論是標準而非規范,且適用于任何經測試的樣品(法定物品)。
‘Standards for an article recognized in the compendia (USP–NF) are expressed in the article's monograph, applicable general chapters, and General Notices. The standards in the relevant monograph, general chapter(s), and General Notices apply at all times in the life of the article from production to expiration.’ ‘Thus, any official article is expected to meet the compendial standards if tested, and any official article actually tested as directed in the relevant monograph must meet such standards to demonstrate compliance’…in all cases, statements about whether the compendial standard is met apply only to the units tested’
在美國藥典-國家處方集(USP–NF)中認可的藥品標準在該藥品的各專論、適用的通則章節以及凡例中闡述。從藥品生產到有效期的整個生命周期內,相關專論、通則章節以及凡例中的標準始終適用。“因此,任何法定藥品如果經過檢測,預期應符合藥典標準,并且任何按照相關專論指示實際檢測的法定藥品必須符合這些標準以證明其合規性”……在所有情況下,關于是否符合藥典標準的表述僅適用于已檢測的藥品單位。
and hence
因此
‘Analytical results observed in the laboratory (or calculated from experimental measurements) are compared With stated acceptance criteria to determine whether the article conforms to compendial requirements.’
在實驗室中觀察到的分析結果(或從實驗測量中計算得出的結果)會與規定的驗收標準進行比較,以確定該藥品是否符合藥典要求。
However, it does discuss sampling in two above 1000 General Chapters; <1010> and <1097>.
然而,它確實在兩篇編號大于 1000 的通則章節(即<1010>和<1097>)中討論了取樣問題。
3.3.2 General Chapter<1010>ANALYTICAL DATA—INTERPRETA TIONAND
TREATMENTviii
通則章節<1010>分析數據——解釋與處理
Effective sampling and randomization are important considerations in mitigating the impact of bias.Sampling is performed after the study has been designed and constitutes the selection of test articles within the structure of the design. How to attain such a sample depends entirely on the question that is to be answered by the data. When possible, use of a random process is considered the most appropriate way of selecting samples.
有效的取樣和隨機化是減輕偏差影響的重要考量因素。取樣工作在研究設計完成之后進行,且取樣是在研究設計架構內對測試物品的選取。如何獲得這樣的樣本完全取決于需要通過數據來解答的問題。在可能的情況下,采用隨機程序被認為是選擇樣本最合適的方式。
The most straightforward type of random sampling is called simple random sampling. However,sometimes this method of selecting a random sample is not desirable because it cannot guarantee equal representation across study factors. The design of a study to release manufactured lots might incorporate factors such as selected times, locations, or parallel manufacturing streams (e.g.,multiple filling lines). In this case a stratified sample whereby units are randomly selected from within each factor can be utilized.
最直接的隨機取樣類型被稱為簡單隨機取樣。然而,有時這種選擇隨機樣本的方法并不理想,因為它無法保證在研究因素中具有同等的代表性。為放行生產批次而進行的研究設計可能會納入諸如選定的時間、地點或并行的生產流程(例如,多條灌裝生產線)等因素。在這種情況下,可以采用分層抽樣,即從每個因素中隨機選擇樣本單元。
Regardless of the reason for taking a sample, a sampling plan should be established to provide details on how the sample is to be obtained to ensure that it is representative of the entirety of the population of interest.
無論出于何種原因進行取樣,都應制定取樣計劃,詳細說明如何獲取樣本,以確保其能代表所關注總體的全部情況。
3.3.3 GeneralChapter<1097>BULK POWDE RSAMPLING PROCEDURES
通則章節<1097>散裝粉末取樣程序
The goals of this chapter are to provide guidance on bulk powder sampling procedures, identify important bulk powder sampling concepts, and collect a knowledge base of useful practices and considerations that can lead to the ideal physical sampling of bulk powder materials.
本章的目的是為散裝粉末取樣程序提供指導,明確重要的散裝粉末取樣概念,并收集有用的實踐和注意事項知識庫,以實現散裝粉末材料的理想物理抽樣。
The primary difficulty in acquiring a representative sample is that the size of the sample for measurement, typically a few milligrams to grams, must be withdrawn from a large population on the order of hundreds to thousands of kilograms. The few milligrams analysed in a laboratory must be taken from a large population of particles in a warehouse in such a manner that the measurement sample is representative of all the particles in the lot.
獲取具有代表性的樣品的主要困難在于,用于測量的樣品規模通常為幾毫克到幾克,而這些樣品必須從數量達數百到數千千克的大量物料中提取。在實驗室中分析的幾毫克樣品,必須以某種方式從倉庫中的大量顆粒物料中獲取,從而使測量樣品能夠代表該批次的所有顆粒。
A typical sampling strategy consists of two basic steps: (1) the primary or gross sample, followed by(2) the secondary sample, which reduces the primary sample to a size that is suitable for laboratory measurement. In short, the goal is to select from the lot a quantity of material suitable for measurement without significantly changing the attribute for which one is sampling.
一個典型的取樣策略包含兩個基本步驟:(1)獲取初始或粗樣,接著(2)獲取二次樣,即將初始樣品縮減至適合在實驗室測量的規模。簡而言之,目標是從批次中選取適合測量的物料數量,同時不會顯著改變進行取樣所針對的屬性。
In parallel with the sample size reduction, sample size calculations must be done in such a way that the sampling strategy has sufficient statistical power to determine whether the attributes of interest lie within the specification ranges with a reasonable degree of certainty. Each step must be done correctly, or the sampling strategy as a whole will not provide a sample that is representative of the
original population.
在減小樣品規模的同時,必須進行樣品量的計算,以使取樣策略具備足夠的統計效力,從而能夠在合理的確定程度下判定所關注的屬性是否處于規定的范圍之內。每一個步驟都必須正確執行,否則作為一個整體的取樣策略將無法提供能夠代表原始總體的樣品。

Figure 3 Sampling strategy that will help to minimize sampling errors
圖 3 有助于將取樣誤差降至最低的取樣策略
3.4 WHO Guidance for sampling of pharmaceutical products and related materials
WHO 藥品及相關物料的取樣指導意見
1. Introduction
介紹
1.1 General considerations
一般考慮因素
1.2 Glossary
術語表
1.3 Purpose of sampling
取樣的目的
1.4 Classes and types of pharmaceutical products and related materials
藥品及相關物料的類別和類型
1.5 Sampling facilities
取樣設備
1.6 Responsibilities for sampling
取樣的職責
1.7 Health and safety
健康與安全
2. Sampling process
取樣過程
2.1 Preparation for sampling
取樣準備
2.2 Sampling operation and precautions
取樣操作及注意事項
2.3 Storage and retention
儲存和保留
3. Regulatory issues
法規問題
3.1 Pharmaceutical inspections
藥品檢查
3.2 Surveillance programmes
監督項目
4. Sampling on receipt (for acceptance)
收貨時取樣(用于驗收)
4.1 Starting materials
起始物料
4.2 Intermediates in the manufacturing process and bulk pharmaceutical products
生產過程中的中間體以及原料藥
4.3 Finished products
成品
4.4 Packaging materials (primary and secondary)
(一級和二級)包裝材料
5. Sampling plans for starting materials, packaging materials and finished products
起始物料、包裝材料和成品的取樣計劃
5.1 Starting materials
起始物料
5.2 Packaging materials
包裝材料
5.3 Finished products
成品
Bibliography
參考資料
Appendix 1 Types of sampling tools
取樣工具的類型
Appendix 2 Sample collection form
樣品采集表
Appendix 3 Steps to be considered for inclusion in a standard operating procedure
附錄 3 納入標準操作規程時需考慮的步驟
Appendix 4 Examples of types of containers used to store samples of starting materials and bulk products
附錄 4 用于儲存起始物料和大宗產品樣品的容器類型示例
Appendix 5 Examples of use of sampling plans n, p and r. See section 6.3 for more details.
3.5 FDA Guidance Investigating Out-of-Specification (OOS) Test Results for
Pharmaceutical Production, May 2022
《2022 年 5 月美國食品藥品監督管理局(FDA)藥品生產中超出規格(OOS)測試結果調查指南》
Retesting and resampling are a critical aspects of this guidance.
重新測試和重新采樣是該指南的關鍵方面
Part of the investigation may involve retesting of a portion of the original sample. The sample used for the retesting should be taken from the same homogeneous material that was originally collected from the lot,tested, and yielded the OOS results. For a liquid, it may be from the original unit liquid product or composite of the liquid product; for a solid, it may be an additional weighing from the same sample composite prepared for the original test.
調查的一部分可能涉及對原始樣品的一部分進行重新測試。用于重新測試的樣品應取自最初從批次中采集、經過測試并得出不合格(OOS)結果的同一均勻物料。對于液體,它可能取自原始的單位液體產品或液體產品的混合樣;對于固體,它可能是從為原始測試準備的同一樣品組合中額外稱取的部分。
3.6 GOOD Samples: Guidance on Obtaining Defensible Samples
良好樣本:獲取可靠樣本的指南
The FDA regulates food as well as drugs, In October 2015, the Sampling and Sample Handling Working Group of the Association of American Feed Control Officials (AAFCO) developed a guidance by a consortium, led by FDA, entitled GOODSamples: Guidance on Obtaining Defensible Samples.This 83 page guidance had the objective to “improve analytical data equivalency among state,federal and local agencies ... Because analytical data is only as good as the quality of thesample”.
美國食品藥品監督管理局(FDA)既監管食品也監管藥品。2015 年 10 月,美國飼料控制官員協會(AAFCO)的取樣和樣品處理工作組在 FDA 的領導下,由一個聯合體制定了一份名為《優質樣本:獲取可靠樣本的指南》的文件。這份長達 83 頁的指南旨在“提高州、聯邦和地方機構之間分析數據的等效性……因為分析數據的質量僅取決于樣本的質量” 。
A subsequent document in 2018, GOOD Test Portions: Guidance On Obtaining Defensible Test Portions (70 pages) was developed and contains detailed information on the TOS in respect of the error structure and sampling methodology for foodstuffs.
隨后在 2018 年的一份文件《優質測試部分:獲取可靠測試部分的指南》(70 頁)得以制定,其中包含了關于食品的測試樣本(TOS)在誤差結構和取樣方法方面的詳細信息。

Figure 4 Categorization of errors contributing to total sampling error and relationship to global estimation error [from figure 4 of reference
圖 4 對導致總取樣誤差的誤差進行分類以及與總體估計誤差的關系
4 Theory of Sampling
抽樣理論
The theory of sampling (TOS) has been developed, primarily by Dr Pierre Gy, between 1950 and the early 2000s. In Gy theory, correct sampling is defined as a sampling scenario in which all particles have the same probability of being included in the sample. The theory was primarily developed for the mining industries and large scale sampling problems of large quantities of bulk materials as to how to generate a representative sample.
抽樣理論(TOS)主要是由皮埃爾·吉博士在 1950 年至 21 世紀初期間提出的。在吉的理論中,正確的抽樣被定義為這樣一種抽樣情形:所有顆粒都有相同的被納入樣本的概率。該理論主要是為采礦業以及大量散裝物料的大規模抽樣問題而發展起來的,旨在解決如何獲取有代表性的樣本這一問題。
These two books are high level monographs and statistically very detailed. More recently, an introductory book has been published by Esbensen.
這兩本書是高水平的專著,并且在統計學方面非常詳盡。最近,埃斯本森出版了一本入門書籍。
A more accessible and less statistical book on the sampling and weighing of bulk solids was published in 1985xvii. In addition, two older books may also be found useful
1985 年出版了一本關于散裝固體抽樣和稱重的書籍,該書更容易理解,且統計學內容較少。此外,還有兩本較早的書籍可能也有用處。
The published literature is sparse with respect to pharmaceuticals and related materials. Representative sampling has been briefly addressedxx to understanding the differences between convenience, target, and self-selected samples. In addition, compositing of samples has also been discussed by Torbeckxxi because compositing samples is appropriate under certain circumstances but raises caveats on how and when it should be applied.
就藥品及相關材料而言,已發表的文獻數量稀少。對于代表性抽樣,只是簡略提及了它對于理解便利抽樣、目標抽樣和自選擇抽樣之間差異的作用。此外,托爾貝克也討論過樣本混合的問題,因為在某些情況下樣本混合是合適的,但在如何應用以及何時應用方面需要注意一些事項。
The best practical application of sampling practices is the WHO guidances for sampling of pharmaceutical products and related materials ix. However, more details regarding the sampling plan sections are given in Chapter 6.
抽樣實踐的最佳實際應用是世界衛生組織關于藥品及相關材料抽樣的指導原則。不過,有關抽樣計劃部分的更多詳細內容在第6 章中給出。
5 Nomenclature of sampling
抽樣的術語
Consistent sampling and testing nomenclature are critical for data integrity and compliance with specifications and regulatory standards. Currently, there is a lack of agreement between the major sources of guidance both within the pharmaceutical industry and between other regulated sectors The International Union of Pure and Applied Chemistry (IUPAC) made nomenclature recommendations for sampling in analytical chemistry in 1990xxii. This paper was referenced in the 2023 IUPAC Orange book.
統一一致的抽樣和測試術語對于數據完整性以及符合規范和監管標準至關重要。目前,制藥行業內部以及其他受監管部門之間的主要指導來源在這方面缺乏共識。國際純粹與應用化學聯合會(IUPAC)在 1990 年就分析化學中的抽樣問題提出了有關術語的建議。2023 年的 IUPAC “橙皮書” 中引用了這篇論文。
ASTM Standard Guide for Defining the Test Result of a Test Method, E2282-14(2019) has also produced a nomenclature guide.
美國材料與試驗協會(ASTM)的《定義測試方法的測試結果的標準指南》(E2282-14(2019))也制定了一份術語指南。
The similarities and differences between literature references have been discussed.
當討論文獻參考之間的異同點時,以下幾個方面可能會被涉及到:
The four key documents for a particular material or product are;
文獻參考資料之間的異同點已被討論過了。
1. Statistical Sampling Plan
統計取樣計劃
2. Sampling Procedure
去樣程序
3. Sampling Protocol
取樣規程
4. Sampling Record
取樣記錄
The relationship between these documents are shown in Figure 1 and some proposed terms and definitions listed in Table 1.
這些文件之間的關系如圖 1 所示,一些提議的術語和定義列于表 1 中。
Table 1 Proposed terms and definitions
提議的術語和定義
|
|
|
|---|---|
|
統計取樣計劃 |
A predetermined procedure for the selection, withdrawal, preservation and preparation of the portions to be removed from a population as samples. - 樣本是從大量材料中選取的任何一部分材料。樣本類型由特定的限定詞來界定,例如隨機樣本,每種樣本類型都有其自身的定義。 A sample is any portion of material selected from a larger quantity of material. The sample type is identified by specific qualifiers, e.g. random sample, with their own definitions. - 其目的是在實際約束條件下,將根據樣本估算出的屬性與批次的實際屬性之間的差異降至最低,并在統計學基礎上限制或控制抽樣操作所產生的不確定性。 The intent is to minimize the difference between the properties as estimated from a sample and the actual properties of the lot, and to limit or control the uncertainty generated by the sampling operation on a statistical basis, all within practical constraints. - 在實際限制條件下,基于統計學原理進行抽樣操作。 sampling operation on a statistical basis, all within practical constraints. - 終點是一個用于測試的具有代表性的樣本,該樣本應能充分反映總體所關注的屬性(特征)。 The end point is a representative sample for testing that can be expected to adequately reflect the properties of interest (Characteristics) of the parent population. |
|
特征 |
A property or attribute of a material that is to be measured or observed. The ISO term is measurand. |
|
抽樣不確定度(均勻性) |
That part of the error associated with using only a fraction of the material population to be sampled and extrapolating to the whole as distinct from analytical or testing error. It arises from a lack of homogeneity in the material population. - 抽樣誤差可以通過對樣本增量進行重復操作以及多次測定來確定,并且(或者)在適當的情況下通過減小顆粒大小來確定。 Sampling error may be determined by replication of sample increments and their multiple determinations or/and by reducing the particle size if appropriate. - 均勻性是指一種屬性、特征或特性在一定數量的材料中均勻分布的程度。非均勻性(均勻性的反面)是抽樣誤差的決定因素。 Homogeneity is the degree to which a property, characteristic or attribute is uniformly distributed throughout a quantity of material. The degree of heterogeneity (the opposite of homogeneity) is the determining factor of sampling error. |
|
抽樣過程(定義) |
Static sampling: The composition of the parent material can be considered as permanent with respect to position in space and stable in time such that the only variable is the sampling position within the space occupied by the consignment, lot or batch. - 動態抽樣:如果母體材料幾乎總是隨時間變化(具有動態性),并且在任何時刻抽取母體材料的一部分都僅反映了該時刻以及特定位置的狀態,例如從混合器中獲取的過程控制樣本。 Dynamic sampling: If the parent material is almost always changing with respect to time (dynamic) and the removal of a portion of the parent material at any instant reflects only a state at that time and at a particular site e.g. In process control samples from a mixer. |
|
|
Sample increment:Each of the discrete, identifiable portions of material removed from a population which can be individually tested. |
|
|
Random sample:The sample so selected that any portion of the population has an equal (or known) chance of being chosen. |
|
|
Representative sample:A sample resulting from a sampling plan. |
|
|
Reference sample:A representative sample of a batch of starting material, packaging material or finished product which is stored for the purpose of being analysed should the need arise during the shelf life of the batch concerned. |
|
|
Retention sample:A sample of a fully packaged unit from a batch of finished product. It is stored for identification purposes. For example, presentation, packaging, labelling, patient information leaflet, batch number, expiry date should the need arise during the shelf life of the batch concerned. |
|
|
Stratified sample:A sample consisting of portions obtained from identified subparts (strata) of the parent population. Within each stratum, the samples are to be taken randomly. |
|
|
Sampling Procedure:The complete sampling operations to be performed on a defined material for a specific purpose. |
|
|
Sampling Protocol:A detailed written description of the sampling procedure to be carried out for a specific consignment, lot or batch. |
|
取樣記錄 |
Documented evidence of the sampling operations carried out on a particular material for a defined purpose. The sampling record should contain the batch number, date and place of sampling, reference to the sampling protocol used, a description of the containers and of the materials sampled, notes on possible abnormalities, together with any other relevant observations, and the name(s) and signature(s) of the sampler(s) in accordance with ALCOA++ principles. [Attributable, Legible, Contemporaneous, Original, Accurate, Complete, Consistent, Enduring, Available, Traceable] |
6 The Sampling Process
抽樣過程
The sampling process is comprised of two distinct phases: design and implementation.
抽樣過程由兩個截然不同的階段組成:設計階段和實施階段。
1. In the design phase, the application of statistical principles is expected to supply a statistical sampling plan to determine the acceptability of a lot, the magnitude of a property, or the quantity of a constituent, within a specified degree of variability with a stated degree of confidence, the document describes,
在設計階段,統計原理的應用旨在提供一個統計抽樣計劃,以便在規定的變異性程度和既定的置信度下,確定一批產品的可接受性、一種特性的量值或者一種成分的數量。該文件對此進行了描述。
2. The implementation phase of sampling involves the physical realization of the statistically designated portions and the removal and preparation of them—an operation easier to state than to perform.
抽樣的實施階段涉及對按統計指定部分的實際獲取,以及對這些部分的提取和準備工作, 這一操作說起來容易,做起來難。
6.1 Statistical Sampling Plans
統計抽樣計劃
A statistical sampling plan is a high-level master document containing all necessary definitions and structures to ensure that a representative sample from a given material population is available for testing in the laboratory. This is a critical document because sampling is always an error-generating process.
統計抽樣計劃是一份高層次的主要文件,它包含了所有必要的定義和架構,以確保能從給定的物料總體中獲取具有代表性的樣本,用于實驗室檢測。這是一份至關重要的文件,因為抽樣始終是一個會產生誤差的過程。
The suggested main terms and definitions are shown in Table 1.
建議的主要術語和定義見表1。
The concept of sampling populations is straight forward, in theory, in that a representative sample is taken from the population and examined for compliance with acceptance criteria be they attributes(e.g. presence of defects) or variables (e.g. dimension within tolerance). On the basis of the finding the level of non-conformances in the representative sample, take a calculated risk of accepting or rejecting the batch (Population) using Acceptance Sampling.
抽樣總體的概念從理論上來說是直接明了的,即從總體中抽取一個有代表性的樣本,并檢查該樣本是否符合驗收標準,這些標準可以是屬性方面的(例如是否存在缺陷),也可以是變量方面的(例如尺寸是否在公差范圍內)。基于對有代表性樣本中不符合項水平的調查結果,運用驗收抽樣方法來承擔接受或拒收該批次(總體)的計算風險。
There will always be a chance that;
總會存在這樣一種可能性:
• good material will be rejected; called the Producer’s risk
合格的材料會被拒收,這被稱為生產方風險。
• bad material will be accepted; called the Consumer’s risk
不合格的材料會被接受,這被稱為使用方(消費者)風險。
6.2 Acceptance Sampling Plans
驗收抽樣計劃
Acceptance sampling plans are available for attributes and variables. The most commonly used in the pharmaceutical industry is for attributes.
驗收抽樣計劃可用于屬性抽樣和變量抽樣。在制藥行業中,最常用的是屬性抽樣計劃。
The mathematical model for sampling without replacement is the hypergeometric distribution whose cumulative distribution function (CDF) is given by
無放回抽樣的數學模型是超幾何分布,其累積分布函數(CDF)由以下給出 :
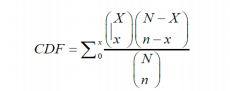
Where xis the number of occurrences of non-conformities found in the sample and nis the number of samples taken from the population. Nis the batch size and Xis the number of non-conformances in it. The () terms are the binomial coefficients.
其中x是在樣本中發現的不合格品的數量,n是從總體中抽取的樣本數量。N是批次大小,X是其中不合格品的數量。( )項是二項式系數。
What is required is an Operational Characteristic (OC) curve based upon a sample size, n,a defined acceptance criterion, (How many defects to accept), and the batch
size.
所需的是一條基于樣本量n、規定的驗收標準(可接受的缺陷數量)以及批次規模的操作特性(OC)曲線。
This OC curve is a plot of the probability of accepting a batch containing a specified % non-conformance (Y axis), based upon the hypergeometric distribution, and the % non-conformances(X axis).
這條操作特性(OC)曲線是基于超幾何分布,以接受含有特定不合格品百分比的批次的概率(縱軸)與不合格品百分比(橫軸)所繪制的圖表。
Fortunately, predetermined ISO standard sampling plans are available for use in laboratories for attributesxxviii and variablesxxix which do not require the calculation of the CDF!
幸運的是,對于屬性抽樣和變量抽樣,實驗室有預先確定的國際標準化組織(ISO)標準抽樣計劃可供使用,這些計劃無需計算累積分布函數(CDF)!
By way of a simple example to illustrate an OC curve, suppose, we have a batch size Nof 1000 items,and decide to take a sample size (n) of 50 intending that we will accept the batch if we have one or less defects after examining n samples otherwise we will reject it.
通過一個簡單的例子來闡釋操作特性(OC)曲線。假設一批產品的批量N為1000件,我們決定抽取樣本量=50。如果在檢驗了n個樣本后,發現的缺陷數量為1個或更少,我們就接受這批產品,否則就拒收該批產品。
We can therefore calculate the OC curve using the CDF function, using Excel, and interpret it.
因此,我們可以使用Excel中的累積分布函數(CDF)來計算操作特性(OC)曲線,并對其進行解釋。

Figure 5 Example Operating Characteristic Curve
圖5 操作特性曲線示例
The AQL with a 95% chance of acceptance gives a producer’s risk of rejection as 0.75%. However,the consumer’s risk is 10 times higher! The LTPD is the percentage defective with a 10% chance of acceptance. This is the probability of falsely accepting a bad lot!
可接受質量水平(AQL)下,接受的可能性為 95%時,生產方被拒收的風險為 0.75%。然而,使用方的風險要高出 10 倍!極限質量水平(LTPD)是指當接受概率為 10%時的不合格品百分比。這就是誤收不良批次的概率!
This guidance provides only a simplified overview of acceptance sampling. There is an excellent book which covers acceptance sampling in great detail.
這份指南僅僅提供了驗收抽樣的一個簡化概述。有一本非常優秀的書籍對驗收抽樣進行了極為詳細的闡述。
In practice, we select a sampling plan based upon a batch size and an acceptance quality limit (AQL)based upon the risk associated with a defect or non-conformance using the sampling tables from ISO 2859-1 for attributes.
在實際操作中,我們根據批次大小以及基于與缺陷或不符合項相關的風險所確定的可接受質量限(AQL),并使用 ISO 2859-1 中針對屬性抽樣的抽樣表來選擇抽樣計劃。
The risk-based strategy depends upon the risk level; Critical, Major or Minor (e.g. cosmetic defects). Let us assume one example for each risk level. The corresponding AQLs for the risk levels are 0.010%, 0.065% and 4.0% respectively. Let us also assume that the corresponding batch sizes are 300,000, 100,000 and 1000.
基于風險的策略取決于風險等級,即關鍵等級、主要等級或次要等級(例如外觀缺陷)。讓我們為每個風險等級舉一個例子。對應這些風險等級的可接受質量限(AQL)分別為 0.010%、0.065%和 4.0%。我們同時假設相應的批次大小分別為 300,000、100,000 和 1000 。

Figure 6 Example AQLs Sample Sizes & Acceptance Criteria
示例可接受質量限、樣本量和驗收準則
The sampling tables do not use exact sample sizes but sample size ranges as an approximation for any given inspection level. In this example, Level II (normal) has been selected which would be the usual practice.
抽樣表并不采用精確的樣本量,而是使用樣本量范圍來近似表示任何給定的檢驗水平。在這個例子中,選擇了檢驗水平 II(正常檢驗水平),這也是通常的做法。
The coloured sample size code letter for the batch size is shown in Figure 6.
針對該批次規模的帶顏色的樣本量編碼如圖 6 所示。

Figure 7 Allocation of sample size range code letter to Level II (normal)
圖7 檢驗水平II(正常檢驗水平)的樣本量范圍編碼分配情況
The accept (Ac)/reject (Re) acceptance numbers depend on the sample size range code letter and the selected AQL. The representative sample sizes to be examined are linked to the sample size range code letter also.
接收(Ac)/拒收(Re)的接收數取決于樣本量范圍編碼字母以及所選的可接受質量限(AQL)。要檢驗的代表性樣本量也與樣本量范圍編碼字母相關聯。

Figure 8 The accept (Ac)/reject (Re) acceptance numbers for inspection Level II (normal) as a function of AQL.
檢驗水平 II(正常檢驗)下,作為可接受質量限(AQL)函數的接收(Ac)/拒收(Re)接收數
However, there is flexibility to switch between reduced and tightened inspection Levels (I & III)depending on the ongoing supplier performance. After 30 conforming lots or batches, a reduction of testing is warranted and reduced inspection level I is able to be implemented. However, if a lot fails then Level II is reinstated. If normal inspection (Level II) operation find that 2 of 5 inspections fail the Level III tightened inspection is implemented. If a total of 5 lots are rejected then inspection should be discontinued until the supplier rectifies the quality issue.
然而,根據供應商的持續表現,可以靈活地在放寬檢驗和加嚴檢驗(檢驗水平I和檢驗水平III)之間切換。當有30批合格產品時,就有理由減少檢驗量,并可實施放寬檢驗水平I。不過,如果有一批產品不合格,就要恢復到檢驗水平II。如果在正常檢驗(檢驗水平II)操作中發現5次檢驗中有2次不合格,就要實施加嚴檢驗水平III。如果總共有5批產品被拒收,那么就應該停止檢驗,直到供應商糾正質量問題為止。

Figure 9 Quality performance; Switching rules
圖 9 質量表現;轉換規則
6.3 WHO guidelines; three reduced sampling plans for starting materials
WHO 針對起始物料的三種簡化抽樣方案
Pages 61 to 93 of this guideline discusses three reduced sampling plans for starting materials; the n,pand rplans. The use of any reduced sampling plan needs to be justified based upon data.
本指南的第61頁至第93頁討論了針對起始原料的三種簡化抽樣方案,即n方案、p方案和r方案。任何簡化抽樣方案的使用都需要有數據作為依據來證明其合理性。
6.3.1 The n plan
The most commonly used plan is the n plan, more commonly called the ‘Root N +1 reduced sampling plan. The statistical aspects have been discussed by Torbeck in 2009. The n plan ‘assumes a uniform material from a recognized source where there is a high degree of confidence in the source ’. However, WHO makes a strong warning about the use of this plan.
最常用的方案是 n 方案,它更普遍地被稱為 “根號 N 加 1 簡化抽樣方案”。托爾貝克在 2009 年探討了該方案的統計學方面的內容。n 方案 “假定所采用的材料來自可認可的來源,且對該來源有高度的信任,并且物料性質均一”。然而,WHO強烈警示了使用該方案的情況。
The n plan is not recommended for use by control laboratories of manufacturers who are required to analyse and release or reject each received consignment of the starting materials used to produce a drug product.
對于那些需要對所接收的用于生產藥品的起始物料進行分析并決定放行或拒收的生產企業的質量控制實驗室而言,不建議使用n方案。
In 5.1.1, it continues;
在 5.1.1 部分,內容繼續寫道:
The “n plan” should be used with great caution and only when the material to be sampled is considered uniform and is supplied from a recognized source. Samples can be withdrawn from any part of the container (usually from the top layer).
“n方案”應極其謹慎地使用,并且只有在待抽樣的物料被認為是性質均一的,且由一個已認可的來源提供的情況下才可使用。樣品可以從容器的任何部位抽取(通常從頂層抽取)。
N is the number of sampling units in the consignment. The value of n is obtained by simple rounding. A minimum number of containers needs to be sampled, e.g. if N is less than or equal to 4, then every container is sampled.
N是該批貨物中的抽樣單元數量。n的值通過簡單的取整得到。需要對一定數量的容器進行抽樣,例如,如果 N 小于或等于 4,則需對每個容器進行抽樣。
6.3.2 The p plan
p 方案
In 5.1.2 it describes the p plan noting the caveat
在 5.1.2 中描述了該計劃,并提到了相關的注意事項。
The “p plan” may be used when the material is uniform, is received from a recognized source and the main purpose is to test for identity. The p plan is based on the formula p = 0.4 *Root N, where N is the number of sampling units. The figures for p are obtained by rounding up to the next highest integer.
當物料性質均一,且來自已認可的來源,同時主要目的是進行鑒別檢驗時,可以使用“p方案”。“p方案”基于公式p=0.4×根號N,其中N是抽樣單元的數量。p的數值通過向上取整到下一個最高整數得到。
According to this plan, samples are taken from each of the N sampling units of the consignment and placed in separate sample containers.
根據這個方案,從該批貨物的 N 個抽樣單元中的每一個抽取樣本,并將其放置在單獨的樣品容器中。
These original samples are transferred to the control laboratory, visually inspected and tested for identity (a simplified method may be used). If the results are concordant, p final samples are formed by appropriate pooling of the original samples.
這些原始樣品被送往質量控制實驗室,進行目視檢查并開展鑒別檢驗(可以使用簡化方法)。如果檢驗結果一致,通過對原始樣品進行適當的合并來形成 p 個最終樣品。
6.3.3 The r plan
r 方案
In 5.1.3, it describes the r plan
在 5.1.3 部分,它描述了 r 方案。
The “r plan” may be used when the material is suspected to be nonuniform and/or is received from a source that is not well known. The r plan may also be used for herbal medicinal products used as starting materials. This plan is based on the formula r = 1.5*Root N, where N is the number of sampling units. The figures for r are obtained by rounding up to the next highest integer.
當物料被懷疑性質不均一,以及/或者物料來自一個不太知名的來源時,可以使用 “r 方案”。“r方案” 也可用于作為起始原料的草藥制品。該方案基于公式 r = 1.5×根號 N ,其中 N 是抽樣單元的數量。r 的數值通過向上取整到下一個最高整數得到。
Samples are taken from each of the N sampling units of the consignment and placed in separate sample containers. These original samples are transferred to the control laboratory and tested for identity. If the results are concordant, r samples are randomly selected and individually subjected to testing. If these results are concordant, the r samples are combined for the retention sample.
從該批貨物的N個抽樣單元中的每一個抽取樣品,并將其放置在單獨的樣品容器中。這些原始樣品被送往質量控制實驗室并進行鑒別檢驗。如果檢驗結果一致,隨機選取r個樣品,并分別對它們進行檢測。如果這些檢測結果一致,則將這 r 個樣品合并作為留樣。
Illustrative examples of these 3 plans are given in Figure 10.
這三種方案的示例在圖10中給出。

Figure 10 Examples of the WHO n, p & r plans for a consignment of 40 container
對于一批包含 40 個容器的貨物,WHO 的 n 方案、p 方案和 r 方案的示例
7 Sampling Procedure & Protocol
取樣程序&方案
The parent material (population) to be sampled is a consignment, batch or lot, uniquely identified,and is specified in the procedure and uniquely recorded as part of the procedure and the protocol.
待取樣的母體物料(總體)是一批貨物、一個批次或一批產品,需進行唯一標識,并在抽樣程序中加以明確規定,且作為取樣程序和方案的一部分進行唯一記錄。
The collection of one or more increments or units taken from a population as required by the material specific sampling procedure and the batch/material specific sampling protocol and suitably identified.
根據特定物料的取樣程序以及特定批次/物料的取樣方案的要求,從總體中采集一個或多個份樣或抽樣單元,并進行適當標識。
The overall sampling process is driven by the sampling protocol and the requirements of the statistical sampling plan (SSP) for a particular material. This protocol contains the complete sampling operations to be performed on a defined material for a specific purpose and is traceable to the SSP.
整個取樣過程由取樣方案以及針對特定物料的統計抽樣計劃(SSP)的要求所驅動。該方案包含了為特定目的而對特定材料需執行的完整取樣操作,并且可追溯至統計抽樣計劃(SSP)。
These increments may be combined (composited) to form a combined sample if specified in the protocol. A sufficient and representative amount sample for testing, of at least twice the amount required for testing, that can be expected to adequately reflect the characteristics of the parent population should be taken.
如果取樣方案有規定,這些份樣可以合并(組成混合樣)。應抽取足夠且具有代表性的樣本用于檢測,其數量至少為檢測所需量的兩倍,且該樣本應能充分反映母體總體的特征。
7.1 In-process sampling
過程中取樣
Much of the sampling effort in companies is focused on materials received and finished products. The importance of a constant structured approach to in-process sampling is often overlooked.
公司的取樣工作大部分集中在進貨物料和成品上。然而,對于過程取樣采用持續的、結構化方法的重要性常常被忽視。
Recently FDA have found it necessary to issue a specific draft guidance in this area.
最近,FDA 認為有必要在這一領域發布一份特定的指導草案。
In section III. GENERAL CONSIDERATIONS FOR IN-PROCESS SAMPLING AND TESTING, it states;
在第三節“過程取樣和測試的一般注意事項”中,其陳述如下:
‘The determination of whether in-process controls, and tests, or examinations meet the regulatory requirements in §211.110 primarily depends on the nature of the drug product (e.g., dosage form) and the type of process used by the manufacturer .’
“對于過程控制、測試或檢查是否符合第 211.110 條的監管要求的判定,主要取決于藥品的性質(例如劑型)以及制造商所采用的生產過程類型。”
And
并且
‘In addition to identifying which critical quality attributes and in-process material attributes to monitor, the manufacturer should define and justify where and when the proposed in-process controls, and testing, or examinations that are used to monitor those attributes should occur.’
“除了確定需要監測的關鍵質量屬性和過程物料屬性之外,制造商還應明確說明并提供合理依據,確定在哪些位置以及何時進行用于監測這些屬性的過程控制、測試或檢查。”
And specifically
具體而言
‘The manufacturer should employ a scientifically sound and appropriate sampling and testing strategy for quality attributes at appropriate points in the process that are adequate to ensure drug product quality. The manufacturer should employ time-based sampling plans for quality attributes, where appropriate.’
制造商應在生產過程的適當環節,針對質量屬性采用科學合理且恰當的取樣和測試策略,以充分確保藥品質量。在適當情況下,制造商應針對質量屬性采用基于時間的取樣計劃。
‘Although in-process controls, and tests, or examinations of in-process materials are required, sampling does not necessarily require steps for physically removing in-process materials to test their characteristics.Innovative technologies allow in-line, at-line, or on-line measurements in place of physical sample removal for laboratory testing.’
“盡管需要對過程物料進行過程控制、測試或檢查,但取樣并不一定需要通過實際取出過程物料來測試其特性。創新技術允許進行在線、線旁或在線測量,以替代取出實物樣品進行實驗室測試。”
‘In batch manufacturing of a solid oral drug product, blend uniformity should typically be assessed before in-process materials continue to the compression step. However, in continuous manufacturing, a manufacturer could conduct sampling and testing at an appropriate point in the process (e.g., at the tablet press feed frame or after compression) to evaluate the adequacy of mixing to ensure batch uniformity and homogeneity.’
“在固體口服藥品的批量生產中,通常應在過程物料進入壓片步驟之前評估混合均勻性。然而,在連續生產中,制造商可以在生產過程的適當階段(例如,在壓片機進料框架處或壓片之后)進行取樣和測試,以評估混合的充分性,從而確保批次的均勻性和均一性。”
8 Sampling Record
取樣記錄
The sampling record is the documented evidence of the actual sampling operations carried out on a particular material for a defined purpose.
取樣記錄是為特定目的對特定物料實施的實際取樣操作的書面證明文件。
The sampling record should contain;
取樣記錄應包含:
• a unique identifier for the parent material
母體物料的唯一識別碼(或唯一標識)
• date, time and place of sampling
取樣日期、時間和地點
• reference to the sampling protocol used and, by inference, the SST
提及所使用的取樣方案,并且由此推斷出統計抽樣計劃(SST)。
• a description of the containers, with labelling instructions, of the materials sampled
對所抽取材料的容器的描述,包括貼標簽的說明。
• the weights of all individual increments or units
所有單個份樣或單元的重量
• If compositing is undertaken, the analytical sample weight is recorded
如果進行了混合操作,要記錄分析樣品的重量。
• notes on possible abnormalities or deviations
關于可能出現的異常情況或偏差的記錄(說明)
• the name and signature of (all) the operator(s) involved.
相關操作人員(全部)的姓名及簽名。
Second person review should ensure traceability and compliance throughout this phase.
應由第二人進行審核,以確保在整個這一階段具有可追溯性且符合規定。

Figure 11 Proposed nomenclature and inputs to the sampling record
擬議的術語以及需錄入取樣記錄的內容
9 Additional Guidance sources on sampling
關于取樣的其他指導資料來源
Eurachem and the Royal Society of Chemistry have resources which provide information on sampling and, in particular, measurement uncertainty as listed below.
歐洲分析化學中心(Eurachem)和英國皇家化學學會(the Royal Society of Chemistry)擁有一些資源,這些資源提供了有關取樣的信息,特別是關于測量不確定度的信息,如下所列。

10 Sampling is only part of the Analysis and Testing Process
取樣只是分析和測試過程的一部分。
Whilst this guidance is concerned with sampling and sample management, it forms only the first, albeit critical, part of the journey. The testing aspect is covered in another guidance document, ECA_AQCG_SOP 03_APLM_v1.0_July 2018.
雖然本指南涉及取樣和樣品管理,但它只是整個流程的第一個部分(盡管是關鍵部分)。測試方面的內容在另一份指南文件《ECA_AQCG_SOP 03_APLM_v1.0_2018 年 7 月》中有所涵蓋。
A high-level process flow is shown in Figure 11.
一個高層次的流程如圖11所示。

Figure 12 Traceability from sample to reportable value
從樣品到可報告數值的可追溯性

來源:Internet


