您當(dāng)前的位置:檢測(cè)資訊 > 科研開發(fā)
嘉峪檢測(cè)網(wǎng) 2022-08-03 23:13
翻譯這篇文章的目的是想讓大家了解一下國外是怎樣做設(shè)計(jì)開發(fā)的,運(yùn)用科學(xué)的方法做研究,這種方法可以運(yùn)用到工藝、清潔程序等開發(fā)中,雖然我們對(duì)本文也不是理解太透徹,但我們很想把這篇文章拋給大家,供大家參考。另:就是TOC法(總有機(jī)碳法)被國外廣泛應(yīng)用到設(shè)備清潔和清潔驗(yàn)證中。
This article examines using experimental design methods to define different procedures for intermediate bulk container cleaning. The authors have evaluated this new approach, in which a highly soluble, low-dose product and a relatively insoluble high-dose product constituted experimental input variables.
本文研究使用實(shí)驗(yàn)設(shè)計(jì)方法來定義中型散裝容器(IBC)的不同清潔程序。作者用高溶解性低劑量產(chǎn)品和相對(duì)溶解性不好高劑量的產(chǎn)品構(gòu)成實(shí)驗(yàn)的輸入變量評(píng)估了這種新方法。
Defining cleaning procedures is crucial to ensuring the elimination of product residues from non-dedicated process equipment. This process can be expensive and challenging, however, in facilities where 30-40 different oral solid-dose products may be manufactured each year.
定義清潔程序?qū)τ诖_保從非專用工藝設(shè)備上消除產(chǎn)品殘留來說是至關(guān)重要的。然而對(duì)于設(shè)備上每年可能會(huì)生產(chǎn)30-40種不同的口服固體制劑產(chǎn)品,這一過程可能是花費(fèi)昂貴并且具有挑戰(zhàn)性的。
Generally, best cleaning procedures are defined based on monitoring final drug content, pH, and the conductivity of water samples for each product until they are within acceptable ranges.
一般來說,最好的清潔程序的定義是基于監(jiān)控每個(gè)產(chǎn)品淋洗水樣的終產(chǎn)品的含量、pH和的電導(dǎo)率,直到它們都在可接受范圍內(nèi)。
This article examines a new way of doing this, using experimental design methods to define different procedures for intermediate bulk container (IBC) cleaning.
本文研究一種新的方式來做到這一點(diǎn),使用實(shí)驗(yàn)設(shè)計(jì)方法來定義中型散裝容器(IBC)的不同清潔程序。
The authors have evaluated this new approach, in which a highly soluble, low-dose product and a relatively insoluble high-dose product constituted experimental input variables.
作者們?cè)u(píng)估了這種新方法,用高溶解性低劑量產(chǎn)品和相對(duì)溶解性不好高劑量的產(chǎn)品構(gòu)成實(shí)驗(yàn)的輸入變量。
Given the number and wide variety of APIs, ingredients, cleaning and processing materials used in pharmaceutical manufacturing, pharmaceutical products could potentially be contaminated with any number of substances. This is why verifying clean liness through cleaning validation is so crucial. Validation identifies potential residues, whether from APIs, ingredients, cleaning agentsand microorganisms, and sets up a process through which potential contaminationis reduced to the lowest acceptable limits (1).
考慮到制藥生產(chǎn)中使用的API,輔料,清潔和加工材料數(shù)量和種類繁多,藥品可能會(huì)受到許多物質(zhì)的污染。這就是為什么通過清潔驗(yàn)證來確認(rèn)清潔是如此關(guān)鍵。驗(yàn)證確定了潛在的殘留物,無論是來自于API,輔料,清洗劑還是微生物,并建立了一個(gè)過程,通過這種過程潛在的污染物被降至最低可接受的限度。
Usually, these limits are defined based on visual, chemical, and microbiological data(2). Chemical limits are expressed as the maximum concentration in the next product (3), amount per surface unit (4), or concentration in the extraction solvent (5). An acceptance limit plus an internal action limit allows pharmaceutical manufacturers to achieve more stringent process control.
通常,這些限度的定義是基于目視,化學(xué),和微生物數(shù)據(jù)。化學(xué)限度表示為在下一產(chǎn)品中的最大濃度,每單位表面積上的數(shù)量,或者萃取溶劑中的濃度。可接受限度加上內(nèi)部行動(dòng)限度使得制藥企業(yè)能夠?qū)崿F(xiàn)更嚴(yán)格的工藝控制。
Once potential residues have been defined, detection methods must be established.Usually, specific methods such as high-performance liquid chromatography (HPLC)(6) and ultraviolet spectroscopy (UV) (7) are used, but non-specific methods such as total organic carbon (TOC) (8, 9) may also be applied, and pH and conductivity determinations should also be used to evaluate the performance ofthe cleaning procedure.
一旦定義了潛在殘留物,必須要建立檢驗(yàn)方法。通常情況下,會(huì)使用專屬性方法,如HPLC和UV,但是非專屬性方法如總有機(jī)碳(TOC)也可能被使用,同時(shí)pH、電導(dǎo)率檢測(cè)也應(yīng)該被用來評(píng)估清潔程序的性能。
Disadvantages of specific methods include the need to develop assays for each new API and validate those methods, a process that can be long and expensive (10, 11). However, TOCcan potentially be applied to any product, is sensitive enough to detect quantities down to μg/L or parts per billion (ppb), and typically involves less time-consuming sample preparation than other methods (12).
專屬性方法的缺點(diǎn)包括需要為每一種新的API開發(fā)檢測(cè)方法并進(jìn)行方法學(xué)驗(yàn)證,這一過程可能是漫長而昂貴的。然而,總有機(jī)碳法(TOC)可被應(yīng)用到任何產(chǎn)品,靈敏度足以使檢測(cè)限達(dá)到μg/L或ppb,相比其他方法會(huì)較少涉及耗時(shí)樣品的制備。
Although specific methods are usually preferred, non-specific techniques can be used if there is a scientific justification for doing so in continuous process monitoring, whereas specific methods could used only for initial validation.
盡管專屬性方法通常是首選的,如果在連續(xù)工藝監(jiān)控中有特定的科學(xué)依據(jù)也是可以使用非專屬性技術(shù)的,專屬性方法只能用于初始驗(yàn)證。
Sampling methods are then selected by assaying rinse water or by surface swabbing (13).Rinse water sampling is used when dealing with very large pieces of equipment or piping, or in situations where there is limited access to equipment surfaces(14). These samples should be correlated with direct swab sampling to be sure that residues are being detected properly and do not remain undissolved oninaccessible equipment surfaces.
取樣方法選擇檢測(cè)淋洗水法或表面擦拭法。在處理非常大的設(shè)備、管道、或者在設(shè)備表面接觸受限的情況下采用淋洗法取樣。涉及采用直接擦拭取樣的樣品情況,應(yīng)確保殘留物能夠被合理檢測(cè)到,并且在接觸不到的設(shè)備表面不會(huì)有未溶解的殘留物。
實(shí)驗(yàn)設(shè)計(jì)簡化操作流程
The authors launched a study to apply the principles of experimental design, and recommendations for cleaning validation, to develop a faster way to set andvalidate procedures for cleaning intermediate bulk containers (IBC) for granulated products. They extended this methodology and applied it to set cleaning validation limits for a vertical granulator in a multiproduct oral solid dosage form facility. The experiments were conducted during the performance qualification step of both of these systems.
作者開展了一項(xiàng)研究,應(yīng)用實(shí)驗(yàn)設(shè)計(jì)原理和清潔驗(yàn)證的建議,開發(fā)一種更快速的方法為顆粒狀產(chǎn)品的IBC容器的清洗來設(shè)定和驗(yàn)證程序。他們擴(kuò)展了這一方法,并將其應(yīng)用于在多產(chǎn)品口服固體制劑設(shè)備中,為立式制粒機(jī)設(shè)定了清潔驗(yàn)證范圍。這些實(shí)驗(yàn)是在這兩種系統(tǒng)的性能鑒定步驟中進(jìn)行的。
Plackett-Burman experimental design methods were used to identify the most important cleaning process parameters, with the minimum number of experimental runs, early in the experimentation phase. To evaluate interactions between variables, a two-level fractional factorial design was conducted after the Plackett-Burman modeling.
Plackett-Burman實(shí)驗(yàn)設(shè)計(jì)(篩選試驗(yàn)設(shè)計(jì))方法用于識(shí)別最重要的清潔工藝參數(shù),在實(shí)驗(yàn)階段的早期,運(yùn)行最少數(shù)量的實(shí)驗(yàn)。為了評(píng)估變量之間的相互作用,在Plackett-Burman模型以后進(jìn)行了二水準(zhǔn)部分階乘實(shí)驗(yàn)設(shè)計(jì)。
Although the equation derived from the two-level factorial design explained the cleaning processes as a third-order equation, the initial Plackett-Burman fitting first-order model (which detected only linear effects) was more valuable inpredicting the process parameters in each step of the cleaning process. The process was scaled up for a vertical granulator, and the cleaning design space was verified.
盡管從二水準(zhǔn)部分階乘實(shí)驗(yàn)設(shè)計(jì)得到的方程式解釋了清潔過程是一個(gè)三階方程,但最初的適用于一階模型的Plackett-Burman在每一步清洗過程中預(yù)測(cè)工藝參數(shù)上更有價(jià)值。這一過程是為了擴(kuò)大立式制粒機(jī)的規(guī)模,清洗設(shè)計(jì)空間也得到了驗(yàn)證。
Results suggest that, although factorial design is useful for understanding process behavior and input variables interactions, Plackett-Burman designs allowed definition of linear models to predict process parameters for 13 new cleaning procedures based on product dose. Five of the cleaning procedures (or recipes), predicted from experimental design, were experimentally confirmed using nine different products. Previous analytical detergent characterization helped todefine the acceptance limits equal to the United States Pharmacopeia(USP) purified water pH and conductivity values.
結(jié)果表明,雖然階乘設(shè)計(jì)對(duì)于理解過程行為和輸入變量的相互作用非常有用,但Plackett-Burman設(shè)計(jì)允許線性模型的定義可以根據(jù)產(chǎn)品劑量來預(yù)測(cè)13個(gè)新的清洗過程的工藝參數(shù)。從實(shí)驗(yàn)設(shè)計(jì)中預(yù)測(cè)的5個(gè)清洗程序(或配方),通過使用9種不同的產(chǎn)品得到了實(shí)驗(yàn)確認(rèn)。先前的分析清潔劑的特性幫助定義了美國藥典(USP)純化的水pH值和電導(dǎo)率值的可接受限度。
The ratio between water volume and equipment surface allowed the procedure to be scaled up and used for granulator cleaning. The design space for the cleaning procedure was also checked in four independent runs for two high-dose products. Results show that the experimentally-derived models provided a high level of assurance that the cleaning procedures met specifications.
用用水量和設(shè)備表面的比例使得該程序規(guī)模得到擴(kuò)大并用于制粒機(jī)的清潔。清潔程序的設(shè)計(jì)空間也在對(duì)兩種高劑量產(chǎn)品的四次獨(dú)立運(yùn)行中被檢測(cè)。結(jié)果表明,實(shí)驗(yàn)得出的模型對(duì)于清潔程序符合規(guī)定提供了高水平的保證。
材料和方法部分
Cleaning stations. Automatic cleaning was conducted in a clean-out-of-place (COP) AISI 304 cabin (WB Model, Cosmec,Italy) for IBC and in a clean-in-place (CIP) system for a 600-L Glatt vertical granulator. A sequential process was used, combining an initial rinse phase to remove larger amounts of adhered product, and a second phase in which detergent was applied. In addition, there were two final rinse steps. Samples of the final rinse water were taken to determine external TOC, pH, and conductivity.The systems were designed to combine, in a synergistic mode, the spectrum of critical cleaning parameters (e.g., time, water, pressure, chemical action and temperature), and were automatically controlled to achieve a robust process(15).
清潔站IBC容器自動(dòng)清潔采取離線清潔(COP)的方式在AISI304艙室中進(jìn)行(WB型,化妝品,意大利)進(jìn)行和600-L的格拉特立式制粒機(jī)系統(tǒng)采取在線清潔(CIP)的方式進(jìn)行。使用了一個(gè)連續(xù)的過程,由初始階段淋洗液來移除大量附著的產(chǎn)品,第二階段在其中加入清潔劑。此外,還有兩個(gè)最后的淋洗步驟。收集最終淋洗水,檢測(cè)離線的總有機(jī)碳(TOC),pH和電導(dǎo)率。該系統(tǒng)的設(shè)計(jì)目的是在一個(gè)協(xié)同的模式下,將關(guān)鍵清洗參數(shù)(如:時(shí)間、水、壓力、化學(xué)反應(yīng)和溫度)的條件合并,并被自動(dòng)控制以達(dá)到一個(gè)穩(wěn)健的過程。
分析方法
TOC was automatically conducted in a GE SIEVERS 900 TOC analyzer, while pH and conductivity were measured in a Mettler-Toledo Seven Multi instrument. Each measurement was repeated at least once. Purified water samples were taken during the last 10 seconds of the final rinse step in ERA ultra-low TOC contentcertified 40-mL TOC flasks.
總有機(jī)碳(TOC)是在GESIEVERS 900系列總有機(jī)碳分析儀中自動(dòng)進(jìn)行檢測(cè)的,而pH和電導(dǎo)率則是用梅特勒-托利多的綜合測(cè)試儀來測(cè)量的。每一次檢測(cè)至少重復(fù)一次。純化的水樣在最后10秒的最后淋洗步驟中被收集到ERA超低TOC含量認(rèn)證的40ml總有機(jī)碳(TOC)燒瓶中。
實(shí)驗(yàn)設(shè)計(jì)
For experimental design definition, version 6.0.1 of Stat-Ease, Inc.’s Design-Expert software was used. A Plackett-Burman design for six factors(i.e., initial rinse volume, detergent volume, detergent concentration, final rinse volume, purified water rinse volume, and product dose) was conducted as 18 runs in a single block, including two central points.
在實(shí)驗(yàn)設(shè)計(jì)的定義中,使用了Stat-Ease, Inc.公司的 Design-Expert軟件的6.0.1版本。Plackett-Burman設(shè)計(jì)的 6 個(gè)因素(即:初始的淋洗體積、清潔劑體積、清潔劑濃度、最終淋洗量、純化水淋洗量和產(chǎn)品劑量)在一個(gè)單一的模塊(包括兩個(gè)中心點(diǎn))中進(jìn)行了 18 次運(yùn)行。
Another two-level fractional factorial design (for the same six factors) was conducted to estimate the effects of all interactions in 18 runs, including two central points. Experiment was conducted as a single block.
另外一個(gè)二水準(zhǔn)部分階乘實(shí)驗(yàn)設(shè)計(jì)(相同的6個(gè)因素)被用來估計(jì)18次運(yùn)行中所有相互作用的影響,包括兩個(gè)中心點(diǎn)。實(shí)驗(yàn)是作為一個(gè)單一的模塊進(jìn)行的。
Both designs were conducted with two different products, used as categorical variables: Product 1 (20-mg dose, highly soluble in water) and Product 2(400-mg dose with low water solubility).
兩種設(shè)計(jì)都是在兩種不同的產(chǎn)品中進(jìn)行的,分別是:產(chǎn)品1(20mg劑量,高水溶性)和產(chǎn)品2(400mg劑量,低水溶性)。
A neutral detergent (Steris’ CIP 300) was used in the experimental phase and during evaluation of the predicted procedures. Steris’ CIP 200 acid detergent and its CIP 100 basic detergent were also evaluated during process performance qualification.
在實(shí)驗(yàn)階段和預(yù)測(cè)過程的評(píng)估中使用了一種中性清潔劑(Steris公司的CIP300)。在工藝性能評(píng)定過程中,還對(duì)Steris公司的CIP200酸清潔劑和CIP 100堿清潔劑進(jìn)行了評(píng)價(jià)。
結(jié)果與討論部分
Cleaning validation constitutes the documented evidence that a cleaning procedure provides equipment ready for manufacturing process, mainly in amultiproduct facility. Activities related to validation studies could be classified in three phases (16). Phase one, commonly called pre-validation, involves research, development, and equipment qualification. Phase two is designed to verify that all the critical parameter limits that have been established are valid, and that the process generates products with sufficient levels of critical quality attributes, even in worst-case situations.
清潔驗(yàn)證是一種文件證據(jù),證明一種清洗程序提供的設(shè)備可以用于生產(chǎn)過程,主要是多產(chǎn)品設(shè)備。與驗(yàn)證研究相關(guān)的活動(dòng)可以分為三個(gè)階段。第一階段,通常叫做預(yù)驗(yàn)證,涉及調(diào)研,開發(fā)和設(shè)備確認(rèn)。第二階段的設(shè)計(jì)是為了驗(yàn)證所有已經(jīng)建立的關(guān)鍵工藝參數(shù)限制都是有效的,并且這個(gè)工藝能夠生產(chǎn)出具有足夠水平的具備關(guān)鍵質(zhì)量屬性的產(chǎn)品,即使在最壞的情況也是如此。
Phase three, the validation maintenance state, involves the frequent review of all documents related to the process performance, to ensure that no deviations, failures, or changes to the production process occurs. A careful design of systems and process controls assures process robustness and quality products.
第三階段,驗(yàn)證維護(hù)狀態(tài),涉及到與工藝性能相關(guān)的所有文檔的周期性審核,以確保不會(huì)出現(xiàn)任何偏差、故障或?qū)ιa(chǎn)工藝的更改。系統(tǒng)和工藝控制的仔細(xì)設(shè)計(jì)確保了工藝的耐用性和高質(zhì)量的產(chǎn)品。
These phases may be applied to cleaning processes, considering that their developmentand validation are based on defining cleaning procedures and controlling analytical assays, acceptable residue limits, critical sampling points, and methods. All of these elements must be established during phase one. In this article, the authors will summarize the process used to establish cleaning procedures for two critical equipment involved in oral solid dose manufacturing in early pre-validation phase.
這些階段可以應(yīng)用于清潔工藝,考慮到他們的開發(fā)和驗(yàn)證是基于定義清潔程序和控制分析含量、可接受殘留限度、關(guān)鍵取樣點(diǎn)和方法。所有這些元素必須在第一階段建立。本文中,作者們將總結(jié)在早期預(yù)驗(yàn)證階段中,為口服固體制劑生產(chǎn)中所涉及的兩個(gè)關(guān)鍵設(shè)備建立清潔程序的過程。
確定合理的殘留限度
In most cases, the first cleaning agent to evaluate in cleaning procedure development is a neutral pH detergent. If it does not provide consistent product removal performance, other acid or basical ternatives are assayed (17). Determining the relationship between detergent dilution in purified water and pH/conductivity values is a prerequisite for using these assays as indicators of cleaning agent removal in cleaning validation studies (18). That is why, in this research, the authors tested three different detergents to evaluate its influence in equipment cleaning procedure performance.
大多數(shù)情況下,在清潔程序開發(fā)中評(píng)估首選的清潔劑是中性pH的清潔劑。如果它不能夠很好的去除產(chǎn)品,其他酸或堿清潔劑將被試驗(yàn)。確定在純化水中稀釋的清潔劑與PH/電導(dǎo)率值之間的關(guān)系是在清潔驗(yàn)證研究中使用這些測(cè)定作為清潔劑去除的指示劑的先決條件。這就是為什么,在這項(xiàng)研究中,作者們測(cè)試三種不同的清潔劑來評(píng)估他們?cè)谠O(shè)備清潔程序性能上的影響。
For the CIP 300 detergent, pH values of different concentrations indicated that pH assays could only detect concentrations exceeding 1000 ppm. For lower levels, pH values corresponded to those of purified water (5.0-7.0). Conductivity values regarding detergent concentration showed that this type of assay could detect concentrations above 10 ppm (1.5 μS/cm-2.7 μS/cm), confirming conductivity as a better assay for determining detergent residues in final rinse water samples than pH measures as previously reported (19).
對(duì)于CIP300清潔劑,不同濃度的pH值顯示pH測(cè)試僅能夠檢測(cè)超過1000ppm的濃度。對(duì)于更低的水平,pH值對(duì)應(yīng)的是純化水的pH(5.0-7.0)。電導(dǎo)率值與清潔劑的濃度有關(guān)顯示,這種類型的分析能夠檢測(cè)的濃度高于10ppm(1.5μS/cm-2.7 μS/cm),確認(rèn)電導(dǎo)率為比pH測(cè)試更好的檢測(cè)清潔劑在最終淋洗水樣品中殘留的分析在之前報(bào)道過。
In the case of the CIP 200 acid detergent, 1 ppm corresponded to the lower limit of water pH (5.20-5.76) and conductivity was in the range of 3.0-3.73 μS/cm. For the CIP100 basic detergent, the 1 ppm pH range slightly exceeded the upper limit water pH (6.92-7.31) and conductivity was between 2.29 and 3.98 μS/cm.
在CIP200的酸清潔劑情況下,1ppm相當(dāng)于水pH的下限(5.20-5.76),電導(dǎo)率在3.0-3.73μS/cm的范圍內(nèi)。對(duì)于CIP100的堿清潔劑,1ppm的pH范圍稍微超過水pH的上限(6.92-7.31),電導(dǎo)率在2.29- 3.98 μS/cm之間。
Taking these results into consideration, USP purified water specification based on pH, conductivity, and TOC were adopted as acceptance criteria to evaluate cleaning process development results. Although these limits below 1 ppm for the three detergents tested is lower than the universally recognized limits of 10 ppm(20), the authors initially considered the potential cumulative effect that residues over surfaces in multiple equipment processes could have on the final product (21). The authors opted for an over dimensioned process to mitigate patient risk, but other factors could justify establishing higher limits.
將這些結(jié)果考慮在內(nèi),采用了基于pH,電導(dǎo)率和總有機(jī)碳(TOC)的USP純化水的規(guī)格被作為可接受限度來評(píng)估清潔工藝開發(fā)的結(jié)果。盡管這三種清潔劑的檢測(cè)限度低于1ppm,低于公認(rèn)的限度10ppm,作者們最初認(rèn)為,在多產(chǎn)品設(shè)備過程中,表面殘留物可能對(duì)終產(chǎn)品有潛在的累積效應(yīng)。作者們選擇了一個(gè)超維度的過程來減少病人的風(fēng)險(xiǎn),但是其他因素能夠證明建立更高限度是合理的。
Plackett-Burman 設(shè)計(jì)結(jié)果
In Plackett-Burman design, the main effects of selected experimental design variables have a complicated confounding relationship with two-factor interactions (22). Therefore, these designs should be used only to study main effects of process parameters when it can be assumed that two-way interactions between them are negligible.
在Plackett-Burman設(shè)計(jì)中,選擇實(shí)驗(yàn)設(shè)計(jì)變量的主要影響是與兩因素相互之間有一個(gè)復(fù)雜的混淆關(guān)系。因此,當(dāng)可以假定雙方之間的相互作用是可以忽略的時(shí)候,這些設(shè)計(jì)應(yīng)該只用于研究工藝參數(shù)的主要影響。
In evaluating the results obtained from Plackett-Burman experiments, a linear model was developed between TOC values and operational cleaning parameters. The Analysis of Variance test showed that the effect dose has a probability value below 0.05, indicating its statistical significance at a confidence level of 95.0% (Table I).
在對(duì)Plackett-Burman實(shí)驗(yàn)結(jié)果的評(píng)價(jià)中,在總有機(jī)碳(TOC)值和操作清洗參數(shù)之間建立了一個(gè)線性模型。方差測(cè)試的分析表明,影響劑量的概率值低于0.05,表明其統(tǒng)計(jì)意義在95.0%的置信水平上(表I)。
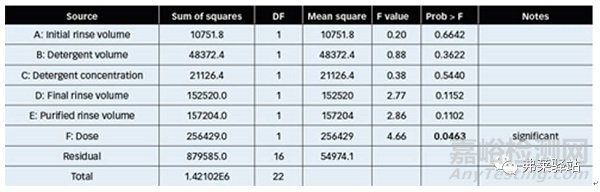
Table I: Variance analysis fortotal organic carbon (TOC) from sample analysis of the Plackett-Burman experimental design. (DF = degrees of freedom; Prob = statistical probability associated with the given F value.)
表1:通過對(duì)Plackett-Burman實(shí)驗(yàn)設(shè)計(jì)的樣品分析得到TOC的方差分析。(DF=自由度;Prob=與給定F值相關(guān)的統(tǒng)計(jì)概率。)
R-squared statistics indicated that the adjusted model explained only 38.102% of the TOC variability. The adjusted R-squared was 14.8902%, suggesting that the linear equation obtained did not completely explain the system’s behavior, so it is likely that higher order interactions take place between the operational variables.
R²統(tǒng)計(jì)表明調(diào)整后的模型只解釋了38.102%的總有機(jī)碳變異性。調(diào)整均方是14.8902%,表明線性方程并沒有完全解釋系統(tǒng)的行為,因此很可能在操作變量之間進(jìn)行更高階的交互。
The Durbin-Watson (DW) statistic was more than 5.0%, proving that there was no serial correlation in residuals. The Plackett-Burman final equation (Equation1), in terms of decoded factors, was:
杜賓-沃森統(tǒng)計(jì)數(shù)據(jù)超過了5.0%,證明了殘差沒有連續(xù)的相關(guān)性。Plackett-Burman的最終方程(方程式1),在解碼因子方面是:
[Eq. 1] TOC—525.397—0.36299• Initial Rinse — 6.15948 •Detergent Volume + 1.52647 • Detergent Concentration — 1.36716 •Final Rinse — 1.38799 • Purified +0.5559804 • Dose
[Eq.1] TOC—525.397—0.36299• 初始淋洗體積 —6.15948 •清潔劑體積 +1.52647 • 清潔劑濃度— 1.36716 •最終淋洗體積— 1.38799 • 純化的淋洗體積 +0.5559804 • 劑量
As expected, this equation indicated that initial rinse, detergent, final rinse,and purified rinse volumes have a negative correlation with TOC values, and increasing dose and detergent concentration also increased the expected TOC results.
正如預(yù)期的那樣,這個(gè)方程式表明,初始淋洗,清潔劑,最終淋洗和純化淋洗體積與總有機(jī)碳值有負(fù)相關(guān),增加劑量和清潔劑濃度也增加了預(yù)期的總有機(jī)碳(TOC)的結(jié)果。
Fractional factorial design results. The low Plackett-Burman adjusted R-squared (14.8902%), indicated that linear equation obtained did not completely explain the system’s behavior. To explain the possible interactions between these variables on TOC variability, a fractional factorial design was completed using the results of the common experiments ofthe Plackett-Burman design. Analysis of variance results are shown in Table II.
部分階乘實(shí)驗(yàn)設(shè)計(jì)的結(jié)果。較低的Plackett-Burman調(diào)整了R²(14.8902%),表明線性方程并不能完全解釋系統(tǒng)的行為。為了解釋這些變量在總有機(jī)碳(TOC)變異性之間可能的相互作用,利用Plackett-Burman設(shè)計(jì)的共同實(shí)驗(yàn)的結(jié)果,完成了一個(gè)部分階乘實(shí)驗(yàn)設(shè)計(jì)。對(duì)方差結(jié)果的分析如表II所示。
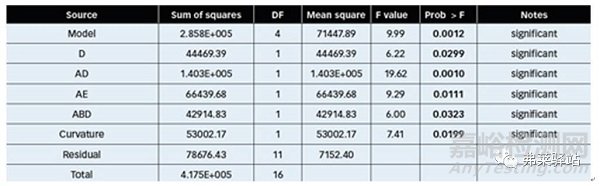

Table II: Variance analysis for total organic carbon (TOC) from sample analysis of two-level factorial experimental design. (DF = degrees of freedom; Prob = statistical probability associated with the given F value.)
表II:從二水準(zhǔn)部分階乘實(shí)驗(yàn)設(shè)計(jì)的樣本分析中得到對(duì)總有機(jī)碳(TOC)的方差分析。(DF=自由度;Prob=與給定F值相關(guān)的統(tǒng)計(jì)概率。)
Values of probability less than 0.0500 indicated significant model terms. In this case, D (Industrial water final rinse), AD (interaction of initial industrial water rinse and Industrial water final rinse), AE (interaction of initial industrial water rinse and purified water final rinse) and ABD (interaction of initial industrial water rinse, detergent volume, and Industrial water final rinse) were significant in model terms. Detergent concentration in the range analyzed was not statistically significant.
概率值小于0.0500表示模型是顯著的。在這種情況下,D(飲用水的最終淋洗體積),AD(初始飲用水的淋洗體積和飲用水的最終淋洗體積之間的相互關(guān)系),AE(初始飲用水的淋洗體積與純化水的最終淋洗體積之間的相互關(guān)系)和ABD(初始飲用水淋洗體積,清潔劑體積和飲用水最終淋洗體積)在模型上是顯著的。在分析范圍內(nèi)的清潔劑的濃度是具有統(tǒng)計(jì)學(xué)意義的。
Adequate precision measures the signal-to-noise ratio. A ratio greater than fouris desirable. The obtained ratio of 10.918 indicated an adequate signal. Residuals were also checked for normality.
足夠的精度測(cè)量信噪比。大于4的比率是可取的。所獲得的10.918的比率表明了一個(gè)充分的信號(hào)。對(duì)殘留物也進(jìn)行了常規(guī)檢查。
The final equation (Equation 2) in terms of decoded factors was as follows:
[Eq. 2] TOC—268.69—55.06● Final Rinse + 97.81 ● Initial Rinse ● Final Rinse + 67.31 ● Initial Rinse ● Purified Rinse—54.09 ● Initial Rinse ● Final Rinse ● Detergen t Volume
最終方程式(方程式2)的解碼因子如下:
[Eq.2] TOC—268.69—55.06●最終淋洗體積+ 97.81●初始淋洗體積●最終淋洗體積+ 67.31●初始淋洗體積● 純化的淋洗體積—54.09● 初始淋洗體積●最終淋洗體積 ●清潔劑體積
This model explains as much of 70.56% of the process variability, and is more exactin describing the influence of process parameters than the linear model obtained from the Plackett-Burman design.
這個(gè)模型解釋了過程變異性的70.56%,比從Plackett-Burman設(shè)計(jì)中獲得的線性模型更準(zhǔn)確的描述了工藝參數(shù)的影響。
IBC容器清潔流程開發(fā)
The Plackett-Burman-derived equation was evaluated for different products doses using Microsoft Excel’s Solver program, in which only process variables were changed. This procedure allowed recipes to be defined for each group of products based on product dose. Because detergent concentration was determined not to be a significant parameter from both experimental designs, it was restricted as a constant to the minimal value assayed. All restrictions were constricted to the range of cleaning process parameters included in the experimental design.
使用Excel的求解程序,Plackett-Burman衍生方程式在不同產(chǎn)品劑量中被評(píng)價(jià),在此過程中,只有過程變量發(fā)生了改變。這個(gè)過程允許根據(jù)產(chǎn)品的計(jì)量為每組產(chǎn)品定義配方。因?yàn)榍鍧崉舛鹊臋z測(cè)不作為實(shí)驗(yàn)設(shè)計(jì)的一個(gè)重要參數(shù),他被限制為一個(gè)常數(shù),以達(dá)到最小值。所有的限制都被限定在實(shí)驗(yàn)設(shè)計(jì)中包含清潔工藝參數(shù)的范圍之內(nèi)。
The resulting procedures were evaluated in at least two containers for seven other products. Results of this testing demonstrated that predicting cleaning process parameters, using the statistically obtained model, was adequate for meeting the acceptance criteria for different doses and for solubility products of batches ranging from 90 to 180 Kg (Table III).
最終的程序在7個(gè)其他產(chǎn)品的至少2個(gè)容器中被評(píng)估。該實(shí)驗(yàn)的結(jié)果表明,使用統(tǒng)計(jì)學(xué)獲得的模型在對(duì)清潔工藝參數(shù)的預(yù)測(cè)中,足以滿足不同劑量、產(chǎn)品批量在90-180kg的可溶解產(chǎn)品的可接受標(biāo)準(zhǔn)。(表III)
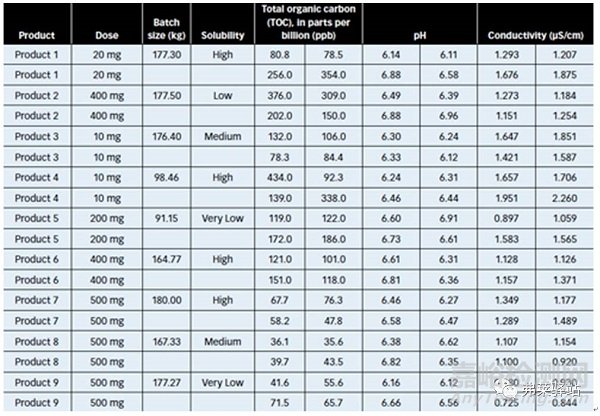
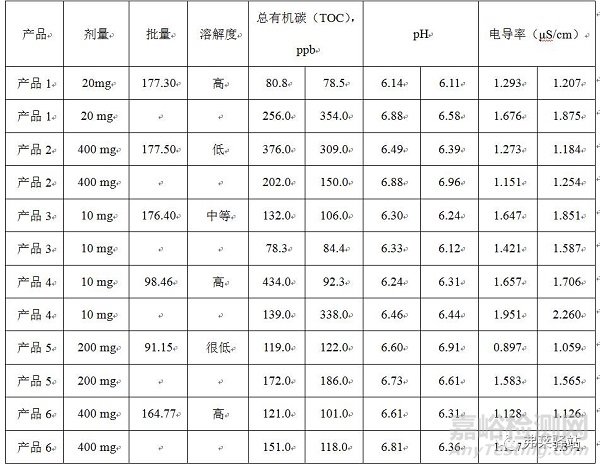
Table III: Results of applying container-cleaning procedures, using recipes developed with Plackett-Burman methods.
表III:應(yīng)用容器清洗程序的結(jié)果,用Plackett-Burman方法開發(fā)的方法。
When used correctly in the right circumstances, visual inspection is a powerful detection method (23, 24). Using the approach outlined in this article, visual inspection of equipment surfaces revealed no residues, suggesting that the method is effective.
在正確的情況下正確使用時(shí),目視檢查是一種強(qiáng)大的檢測(cè)方法。使用本文所述的方法,對(duì)設(shè)備表面的目視檢查要做到?jīng)]有發(fā)現(xiàn)殘留物,才表明該方法是有效的。
Cleaning procedures developed for product dose groups allowed for reducing process time because the worst-case cycle approach requires 11 minutes. For lower doses, the process times were as follows:
Product 3-10 mg: 6 minutes
Product 1-20 mg: 7 minutes
Product 5-200 mg: 8 minutes
Product 2-400 mg: 9 minutes.
為產(chǎn)品劑量組開發(fā)清潔程序允許減少時(shí)間過程,因?yàn)樽顗那闆r的循環(huán)方法需要11分鐘。對(duì)于較低的劑量,過程時(shí)間如下:
產(chǎn)品劑量3-10mg:6分鐘
產(chǎn)品劑量1-20mg:7分鐘
產(chǎn)品劑量5-200mg:8分鐘
產(chǎn)品劑量2-400mg:9分鐘
The Plackett-Burman model was more practical because it allowed the authors to define operational parameters for 13 groups of products based only on the results obtained with two products of different dose and solubility. This method could be easily applied in situations where new automatic systems are installed in a facility to establish cleaning process conditions faster and at lower cost than developing cleaning process conditions for each product separately.
Plackett-Burman模型更實(shí)用,因?yàn)樗试S作者們根據(jù)不同劑量和溶解度的兩種產(chǎn)品得到的結(jié)果,為13組產(chǎn)品定義操作參數(shù)。這種方法可以很容易的應(yīng)用于在設(shè)備安裝的新自動(dòng)化系統(tǒng),以更快的建立清潔工藝條件,并且比單獨(dú)為每一個(gè)產(chǎn)品開發(fā)清潔工藝條件的成本更低。
Other critical elements that should be validated are the maximum delay before cleaning (i.e., the maximum amount of time that the equipment should be dirty before it is cleaned (25) and the maximum time that it could remain clean after the applied procedure (26). These values could be included as input and output variables, respectively, in the experimental design.
其他需要驗(yàn)證的關(guān)鍵因素是清洗前的最大延遲(即:設(shè)備在清洗前保持未清潔的最大時(shí)間和應(yīng)用程序后保持清潔的最大時(shí)間。在實(shí)驗(yàn)設(shè)計(jì)中,這些數(shù)值應(yīng)該分別被包括在輸入和輸出變量中)。
制粒機(jī)清潔流程開發(fā)
In order to speed development of a cleaning procedure for a vertical granulator, a relationship between the granulator and the container product contact surface area and cleaning volumes was calculated. This approach facilitated the process of develop and evaluating cleaning recipes previous to validation.
為了加快立式制粒機(jī)的清潔程序的開發(fā),計(jì)算了制粒機(jī)和容器的產(chǎn)品接觸表面積和清潔體積之間的關(guān)系。這種方法促進(jìn)了開發(fā)和評(píng)估在進(jìn)行驗(yàn)證之前的清潔配方的過程。
For scale-up, a ratio was obtained between the total volume of water required to clean 500-mg dose containers and the container area. This value was related to the correspondent 600-L granulator product contact area, and the relationship used to determine the volume required for cleaning.
為了擴(kuò)大規(guī)模放大產(chǎn)品,在清洗500mg劑量的容器所需的總水量和容器面積之間得到一個(gè)比率。這個(gè)值與600L的制粒機(jī)產(chǎn)品接觸面積有關(guān),這種關(guān)系用于確定清潔所需的體積。
The cleaning procedure was adjusted sequentially, in order to reach the total calculated volume. This volume was then used as a central point, and variation around it was determined, using a fractional factorial design for three variables with no central point.
清洗程序按照順序進(jìn)行調(diào)整,為了達(dá)到總計(jì)算體積。這個(gè)體積隨后被用作中心點(diǎn),并確定了圍繞它的變量,使用了一個(gè)沒有中心點(diǎn)的3個(gè)變量的部分階乘設(shè)計(jì)。(部分析因設(shè)計(jì))
Two categorical variables were used (two different 500-mg products and two different detergents). Robustness of the design space for the cleaning procedure was determined in eight runs with two different 500 mg product doseand CIP 200 and CIP 100 detergents.
使用兩種分類變量(兩種不同的500mg產(chǎn)品和兩種不同的清潔劑)。清洗程序的設(shè)計(jì)空間的耐受性是在用兩種不同的500mg產(chǎn)品劑量和CIP200,CIP100清潔劑的8次運(yùn)行中確定的。
Results indicated (see Table IV) that in all cases, actual residue limits were below the levels calculated, and were in the parts-per-million of TOC for Product 7 and Product 8.
結(jié)果表明(見表IV)在所有情況下,實(shí)際殘留限度低于計(jì)算的水平,并且在產(chǎn)品7和8的總有機(jī)碳的每百萬分中(ppm)。
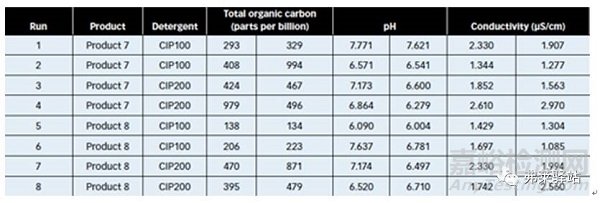

Table IV: Results of a design space cleaning procedure developed for a 600-L Glatt vertical granulator, using the ratio of water volume to equipment surface. CIP is clean-in-place. Visual inspection confirmed that the procedure development was robust. No residues could be detected on any of the equipment surfaces.
表IV:清洗程序空間設(shè)計(jì)的結(jié)果,該程序是為一種600L的格拉特立式制粒機(jī)開發(fā)的,用了水體積和與設(shè)備表面積的比率。目視檢查證明了該程序的開發(fā)是耐用的。任何設(shè)備表面都不能檢測(cè)到殘留物。
結(jié)論部分
Evaluations suggest that the mathematical model obtained from a Plackett-Burman design can predict, with a high degree of accuracy, the optimal conditions for the processof IBC cleaning. Results using the model were checked in nine products in atleast two independent replicates. In all cases, the results were obtained within the acceptance limits of product and detergent removal.
評(píng)估表明,從Plackett-Burman(篩選試驗(yàn)設(shè)計(jì))得到的數(shù)學(xué)模型能夠準(zhǔn)確地預(yù)測(cè)IBC清洗過程的最佳條件。使用該設(shè)計(jì)模型的結(jié)果在9個(gè)產(chǎn)品至少兩個(gè)獨(dú)立的重復(fù)中被檢查。在所有情況下,這些結(jié)果都是在產(chǎn)品和清潔劑去除的可接受范圍內(nèi)得到的。
Application of different cleaning process parameters (in terms of rinse and detergent volumes) for each product allowed water consumption to be reduced by 320 L per container, resulting in better use of installed capacities for clean-out-of-place cleaning of IBCs, because each type of product had a defined process time. If the common approach of determining the “worst-case” procedureis used for all containers, time and water wastes are significantly increased.
每一種產(chǎn)品的不同清潔工藝參數(shù)(淋洗和清潔劑體積)的應(yīng)用允許每個(gè)容器的耗水量減少至320L,源于更好地利用了IBC的離線清洗組裝能力,因?yàn)槊恳活愋偷漠a(chǎn)品都有一個(gè)確定的工藝時(shí)間。如果確定最壞情況程序的常用方法被用于所有的容器,時(shí)間和水的浪費(fèi)會(huì)顯著增加。
When the process was scaled up to clean a vertical granulator, results of the predicted design space were higher than those obtained for IBC, but all results were below 10 ppm, demonstrating that experimental design can be a powerful tool for developing more robust cleaning procedures.
當(dāng)這個(gè)過程被擴(kuò)大規(guī)模來清洗立式制粒機(jī)時(shí),可預(yù)測(cè)性的設(shè)計(jì)空間結(jié)果會(huì)比IBC要高,但是所有的結(jié)果都低于10ppm,表明實(shí)驗(yàn)設(shè)計(jì)可以成為一個(gè)強(qiáng)有力的工具來開發(fā)更耐用的清潔程序。

來源:制藥技術(shù)


