您當前的位置:檢測資訊 > 生產品管
嘉峪檢測網 2025-05-09 13:40
近日,PDA在PDA-letter上發表了關于膠塞斗的滅菌的文章——《對間接與產品接觸的表面進行滅菌處理所面臨的挑戰》,該文章聚焦無菌生產中間接與產品接觸的膠塞斗滅菌問題,對比可拆卸與不可拆卸膠塞斗的滅菌方式進行的風險分析并給出建議:
現狀,33% 的企業膠塞斗不拆卸或過重,不滿足歐盟 GMP 附錄 1 要求,如使用替代程序需經質量風險管理驗證。
符合歐盟藥品生產質量管理規范(GMP)附件 1 的最簡單方法是使用可拆卸的膠塞斗,并且新的灌裝線應滿足這一要求。或者,對不可拆卸部件的生產線進行重新設計也是一種選擇,不過對于某些公司來說,這可能成本過高。每個制造商都應根據自身的操作程序和已實施的控制措施選擇合適的程序。
原文及翻譯如下
Challenges in Sterilizing Indirect Product-Contact Surfaces
對間接與產品接觸的表面進行滅菌處理所面臨的挑戰
For aseptic processes, EudraLex GMP Annex 1: Manufacture of Sterile Medicinal Products, requires that direct and indirect contact parts are sterilized.
對于無菌生產工藝,歐盟藥品管理法規《歐盟藥品 GMP 附錄 1:無菌藥品的生產》要求對(與產品)直接和間接接觸的部件進行滅菌處理。
Based on the current historical design of some filling lines, however, certain stopper bowls are either fixed, built-in or extremely heavy and not possible to remove for offline cleaning and sterilization. Facilitating the removal of these stopper bowls would require a significant redesign of existing lines. Therefore, a risk-based approach is needed to understand the risk of contamination during the preparation of the stopper bowl for aseptic production. Appropriate measures and controls should be developed and included in the company contamination control strategy document. This article proposes a risk assessment of two scenarios for the preparation of the stopper bowl—when it can be dismantled and when it cannot be dismantled. This article focuses on recommended procedures based on risk assessment and measures to mitigate the risk of contamination during aseptic production in both scenarios.
然而,基于目前一些灌裝生產線的傳統設計,某些膠塞斗要么是固定的、內置的,要么極其沉重,無法拆卸下來進行離線清潔和滅菌。要使這些膠塞便于拆卸,需要對現有的生產線進行重大重新設計。因此,需要采用基于風險的方法,來了解在為無菌生產準備膠塞的過程中存在的污染風險。應該制定適當的措施和控制方法,并將其納入公司的污染控制策略文件中。本文針對膠塞的準備工作提出了兩種情況的風險評估 —— 即膠塞能夠拆卸和不能拆卸的情況。本文重點介紹了基于風險評估的推薦程序,以及在這兩種情況下降低無菌生產過程中污染風險的措施。
Background
背景
According to a survey conducted in 2023 to more than 65 companies from around the world, 67% have a detachable stopper bowl, while the remaining 33% have a stopper bowl that is either not detachable or too heavy to be dismantled for cleaning and sterilization. Of these companies, 28% are located in the United States and Canada, 11% in Latin America (Brazil and Mexico), 8% in Asia (India, Korea, and Indonesia), 3% in South Africa and 36% in Europe (Belgium, Germany, Spain, Switzerland, Netherlands, Denmark, Ireland, Italy, the United Kingdom and France) (1).
根據 2023 年對來自全球 65 多家公司進行的一項調查,67% 的公司擁有可拆卸的膠塞(塞子),而其余 33% 的公司的膠塞要么不可拆卸,要么過于沉重,無法拆卸下來進行清潔和滅菌處理。在這些公司中,28% 位于美國和加拿大,11% 位于拉丁美洲(巴西和墨西哥),8% 位于亞洲(印度、韓國和印度尼西亞),3% 位于南非,36% 位于歐洲(比利時、德國、西班牙、瑞士、荷蘭、丹麥、愛爾蘭、意大利、英國和法國)(1)。
The survey shows that, as of 2023, there was a considerable percentage (33%) of manufacturers that do not comply with the requirements of the EU GMP Annex 1 2022 updates. For any newly installed filling lines, the expectation is clear that all direct and indirect product-contact parts should be able to be removed to be cleaned and sterilized offline. On the other hand, the implementation of alternative procedures differing from the recommendation in EU GMP Annex 1 will need to be justified with the tools of quality risk management (QRM) (2).
該調查顯示,截至 2023 年,有相當比例(33%)的制造商不符合歐盟藥品生產質量管理規范(GMP)附錄 1 在 2022 年更新的要求。對于任何新安裝的灌裝生產線,明確的期望是所有與產品直接和間接接觸的部件都應該能夠拆卸下來,以便進行離線清潔和滅菌。另一方面,實施與歐盟 GMP 附錄 1 中的建議不同的替代程序,需要使用質量風險管理(QRM)工具來證明其合理性(2)。

Figure 1 The survey indicates 67% of companies have a detachable stopper bowl and around 33% have stopper bowls which are either not detachable or too heavy to be washed out of place
圖 1 該調查表明,67% 的公司擁有可拆卸的膠塞,大約 33% 的公司的膠塞要么不可拆卸,要么過于沉重,無法拆卸下來進行清洗。
The analysis below utilizes the Failure Mode and Effect Analysis risk-assessment tool to identify and assess the different risks associated with detachable and nondetachable stopper-bowl scenarios. The goal of this exercise will be to see what the major challenges in both are and what would be the best recommendations in each case. Many assumptions are made along the way to assess both situations, such as a certain facility design or a positive pressure isolator as barrier technology, and manufacturers might arrive at a different conclusion depending on the design of their facilities, barrier technologies, controls and procedures in place. Further, the analysis will be done for the stopper bowl as a challenging item due to its size, but it is applicable to any other indirect surface in the filling line.
下面的分析使用故障模式與影響分析風險評估工具,來識別和評估與可拆卸和不可拆卸膠塞情況相關的不同風險。這項分析工作的目標是了解這兩種情況的主要挑戰,以及在每種情況下的最佳建議。在評估這兩種情況的過程中做了許多假設,例如特定的設施設計或作為隔離技術的正壓隔離器,并且制造商可能會根據其設施設計、隔離技術、控制措施和現有程序得出不同的結論。此外,由于膠塞的尺寸原因,將其作為一個具有挑戰性的項目進行分析,但該分析適用于灌裝生產線中的任何其他間接表面。
Before starting with the assessment of the two scenarios, a brief introduction to risk management methodology and the different scales defined to assess the severity, probability and detectability will be presented.
在開始評估這兩種情況之前,將簡要介紹風險管理方法,以及為評估嚴重程度、發生概率和可檢測性而定義的不同等級。
Risk Management Methodology
風險管理方法
Risk assessments consist of the (3):
風險評估包括以下內容(3):
Identification of hazards
危害識別
Analysis
分析
Evaluation of risks associated with exposure to those hazards
對暴露于這些危害所相關的風險進行評估
To identify the risk(s) within the lifecycle of a stopper bowl, three questions are asked:
為了識別膠塞生命周期內的風險,需要提出三個問題:
What might go wrong?
可能會出現什么問題?
What is the likelihood (probability) it will go wrong?
出現問題的可能性(概率)有多大?
What are the consequences (severity)?
后果是什么(嚴重程度如何)?
The ability to detect the harm (detectability) was factored into the estimation of risk. The overall risk was quantitatively determined by multiplying the values of probability x severity x detectability for each identified risk. To quantify the overall risk, scales were defined to assess the severity, probability and detectability (3).
危害的可檢測能力(可檢測性)被納入風險評估的考慮因素。通過將每個已識別風險的概率、嚴重程度和可檢測性的值相乘,來定量確定總體風險。為了量化總體風險,定義了評估嚴重程度、概率和可檢測性的等級(3)。
Severity
嚴重程度
The severity is defined as a measure of the possible consequences of a hazard (3). The scale proposed uses the following aspects to determine the level of severity:
嚴重程度被定義為對危害可能產生的后果的一種衡量標準(3)。所提出的等級標準利用以下幾個方面來確定嚴重程度等級:
Probability of risk to product quality and to patient that could lead to partial or total batch rejection
對產品質量和患者存在的風險概率,這種風險可能導致部分或整批產品被拒收
Presence of further steps that can help reduce the associated risk
是否存在有助于降低相關風險的后續步驟
Proximity to the final filling of product
距離產品最終灌裝環節的接近程度
Table 1 Severity scale proposed for the risk assessment
表 1 風險評估所提出的嚴重程度量表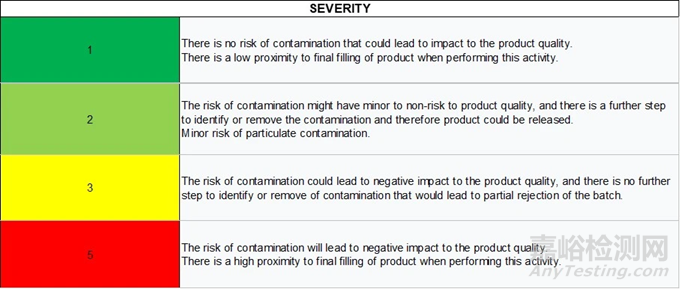
Probability
可能性
The probability scale is defined in Table 2. The evaluation should be based on:
可能性等級在表 2 中進行了定義。評估應基于以下方面:
Data collected through previous experience in the facility
通過工廠先前經驗收集的數據
Data coming from environmental monitoring (EM) and particle monitoring
來自環境監測(EM)和顆粒監測的數據
Data from previous deviations and investigations
來自先前偏差情況和調查的數據
Capability assessment if there is enough data
如果有足夠數據的話,進行能力評估
Historical knowledge and published literature
歷史知識和已發表的文獻
Level of automation—In case of a manual step, how many manual steps are needed and what is the complexity of the manual steps to complete the task?
自動化水平 —— 如果是手動步驟,完成任務需要多少手動步驟,以及手動步驟的復雜程度如何?
As defined in Table 2, a rating of 1 means that, during the activity evaluated, it is not likely that microbial, particle or chemical contamination will occur. The type of contamination will depend on the step evaluated. In a cleaning step, for example, the risk is mainly of chemical contamination or cross-contamination. To assess the probability, if there is enough data for a capability analysis, this can be applied. If this is not the case, the scale in Table 2 suggests other options for the evaluation, such as by the level of automation, experience or published literature.
如表 2 中所定義的,評分為 1 意味著在評估的活動過程中,不太可能發生微生物、顆粒或化學污染。污染的類型將取決于所評估的步驟。例如,在清潔步驟中,風險主要是化學污染或交叉污染。為了評估可能性,如果有足夠的數據進行能力分析,就可以采用這種方式。如果沒有足夠數據,表 2 中的等級給出了其他評估選項,比如依據自動化水平、經驗或已發表的文獻來評估。
For example, we can evaluate the cleaning step for the stopper bowl considering a common, yet challenging, residue like silicone oil.
例如,我們可以考慮像硅油這樣常見但具有挑戰性的殘留物,來評估膠塞的清潔步驟。
If the stopper bowl is fixed, the cleaning step is done manually. According to Table 2, the probability can be assigned with a rating of 3 or even of 5 depending on how complex this activity is to be performed. This will depend on the size of the bowl and the design of the line.
如果膠塞是固定的,清潔步驟需手動完成。根據表 2,根據這項活動執行的復雜程度,可能性的評分可以是 3,甚至是 5。這將取決于膠塞的大小和生產線的設計。
If the stopper bowl is detachable, the probability of having issues with the cleaning is lower. The cleaning is usually done in a parts washer using a validated process. The use of laboratory coupon studies can help in designing a robust cleaning process, which will ensure the removal of silicone oil. In this case, the probability of having an unsuccessful cleaning is a rating of 1.
如果膠塞是可拆卸的,清潔出現問題的可能性較低。清潔通常在零件清洗機中使用經過驗證的工藝來完成。利用實驗室試片研究有助于設計一個可靠的清潔工藝,這將確保硅油被清除。在這種情況下,清潔不成功的可能性評分為 1。
Table 2 Probability scale proposed for the risk assessment
表 2 風險評估所提出的可能性等級
Detectability
可檢測性
The detectability has to do with the controls in place to detect process failures. Controls that can be in place in a cleanroom are:
可檢測性與用于檢測工藝故障的現有控制措施有關。潔凈室中可能采用的控制措施包括:
Particle monitoring
顆粒監測
Microbial monitoring or EM
微生物監測或環境監測(EM)
Microbial monitoring through contact plate or settle plate
通過接觸碟或沉降碟進行微生物監測
Supervision of operators during procedures
在操作過程中對操作人員進行監督
Four-eyes principle
雙人復核原則(四眼原則)
Visual inspection
目視檢查
Continuous monitoring of critical variables
對關鍵變量進行持續監測
Table 3 shows the proposed scale for detectability. The evaluation should be done based on how good the controls in place are to detect the risk at the moment something is not performed as expected. For example, during manual material transfer, the detectability can be assigned a rating of 5 since it is very hard to detect a failure in the process leading to microbial contamination.
表 3 展示了所提出的可檢測性等級。評估應基于現有控制措施在檢測某事物未按預期執行時風險的能力有多強。例如,在手動物料轉移過程中,可檢測性可以評定為 5 分,因為很難檢測到導致微生物污染的工藝故障。
Table 3 Detectability scale proposed for the risk assessment
表 3 風險評估所提出的可檢測性等級
Risk Priority Number
風險優先數
The risk priority number (RPN) can be calculated as the multiplication of the probability, severity and detectability of each step. Table 4 shows a proposed scale for the overall risk classification according to the obtained RPN. Based on this scale, the risk can be determined as low, moderate, or high. It can be noted that, from a certain RPN, control measures are needed to reduce the risk. By doing this, only the detectability is going to be impacted. The severity and probability of the risk of the step should remain the same unless a substantial change is done to the process step.
風險優先數(RPN)可以通過將每個步驟的可能性、嚴重程度和可檢測性相乘來計算。表 4 展示了根據所獲得的風險優先數(RPN)提出的總體風險分類等級。基于這個等級,風險可以被確定為低、中或高。需要注意的是,從某個風險優先數(RPN)來看,需要采取控制措施來降低風險。通過這樣做,只有可檢測性會受到影響。除非對工藝步驟進行重大更改,否則該步驟風險的嚴重程度和可能性應保持不變。
Table 4 Overall risk classification according to the risk priority number
表 4 根據風險優先數劃分的總體風險分類
Considerations – Facility Design and Barrier Technology
注意事項 - 設施設計和隔離技術
To assess the risks in each scenario, each step of the lifecycle of the stopper bowl will be analyzed.
為了評估每種情況下的風險,將對膠塞斗生命周期的每個步驟進行分析。
For the purpose of explaining the risks and control measures associated with the stopper bowl within its lifecycle, Figure 2 displays an example of the configuration of a facility. There are two different layouts—with (A) and without (B) a double-door autoclave—that will only impact the way in which the transfer process of the stopper bowl and other indirect surfaces is performed in Scenario 1, as will be explained. There are dedicated carts for Grade D and Grade C areas. An isolator with automated vaporized hydrogen peroxide (VHP) biodecontamination is considered the barrier technology available.
為了解釋膠塞在其生命周期內的風險和控制措施,圖 2 展示了一個設施配置的示例。存在兩種不同的布局 —— 配備(A)和不配備(B)雙門高壓滅菌器 —— 正如將要解釋的那樣,這只會影響在場景 1 中膠塞和其他間接表面的轉移過程的方式。有專門用于 D 級和 C 級區域的推車。帶有自動汽化過氧化氫(VHP)生物凈化功能的隔離器被視為可用的隔離技術。
(A) Double-door autoclave
(A)雙門高壓滅菌器

Figure 2 Double-door autoclave
圖 2 雙門高壓滅菌器
(B) Without double-door autoclave
B)無雙門高壓滅菌器

Figure 3 Two different cleanroom layouts area—with and without double-door autoclave
圖 3 兩種不同的潔凈室布局 —— 配備和不配備雙門高壓滅菌器
Scenario 1: Stopper Bowl is Detachable
場景 1:膠塞是可拆卸的
A typical lifecycle of the stopper bowl for this scenario can be seen in Figure 4.
在這種場景下,膠塞的典型生命周期如圖 4 所示。
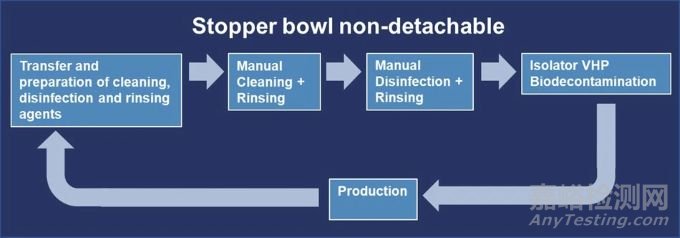
Figure 4 Lifecycle of a detachable stopper bowl
圖 4 可拆卸膠塞的生命周期
Cleaning of the Stopper Bowl
膠塞的清潔
If the stopper bowl is detachable, it is usually cleaned with a parts washer. This eliminates the risk of poor reproducibility, which must be considered for scenario 2 with non-detachable stopper bowl.
如果膠塞是可拆卸的,通常會使用零件清洗機進行清洗。這就消除了重現性差的風險,而對于場景 2 中不可拆卸的膠塞,就必須考慮這種風險。
The risk within the cleaning process is associated with:
清潔過程中的風險與以下因素有關:
How hard is the residue to clean?
殘留物的清潔難度如何?
How is the cleaning procedure designed?
清潔程序是如何設計的?
The harder it is to clean the residues, the higher the risk of the cleaning process failing. Residues of silicone oil coming from siliconized stoppers are quite common, as are hard-to-clean residues due to their poor solubility in water. The use of formulated chemistries that have different components in the formulation, such as surfactants, chelants and binders, will provide more effective cleaning for hard-to-clean soils (4). An appropriate cleaning procedure, its design based on laboratory coupon studies to support the selection of the right chemistry, concentration, temperature and time, will reduce the risks of failed procedures (4). Further, silicone residue is hard to detect via visual inspection and often not detected by operators. Easier-to-clean and easier-to-detect residues might reduce the risk within this step.
殘留物越難清潔,清潔過程失敗的風險就越高。來自硅化膠塞的硅油殘留物很常見,由于其在水中的溶解性差,這些殘留物很難清潔。使用配方中含有不同成分(如表面活性劑、螯合劑和粘合劑)的化學制劑,將對難以清潔的污漬提供更有效的清潔(參考文獻 4)。基于實驗室試片研究設計適當的清潔程序,以支持對正確的化學制劑、濃度、溫度和時間的選擇,這將降低清潔程序失敗的風險(參考文獻 4)。此外,硅酮殘留物很難通過目視檢查發現,而且操作人員往往無法檢測到。更容易清潔和更容易檢測到的殘留物可能會降低這一步驟中的風險。

Figure 5 Risk evaluation of the cleaning step of a detachable stopper bowl
圖 5 可拆卸膠塞清潔步驟的風險評估
Sterilization Wrapping – Preparation of the Stopper Bowl for Steam Sterilization
滅菌包裝 —— 膠塞的蒸汽滅菌準備
The risk of this step is highly dependent on the wrapping material used. The manipulation from operators can also lead to particle and microbial contamination, though it is considered that the operators are well-trained in aseptic processing. Thus, this risk is lower in comparison to contamination coming from the wrapping material.
這一步驟的風險在很大程度上取決于所使用的包裝材料。盡管認為操作人員在無菌操作方面訓練有素,但操作人員的操作也可能導致顆粒和微生物污染。不過,與來自包裝材料的污染相比,這種風險較低。
If Tyvek is used, the risk of particles is considered low, since peeling open a sealed Tyvek pouch generates less than one-tenth the number of particles (0.5 µ and 5 µ in size) compared to a cellulose pouch with a rating of 5. The probability of risk of particles would be a rating of 1. Severity is considered a rating of 3, as this can affect the product quality although not as severe as for a product rejection. Detectability is considered as a rating of 3 through particle monitoring, for example, giving an overall RPN=9, which falls in the green area.
如果使用特衛強(Tyvek)材料,顆粒污染的風險被認為較低,因為與評級為 5 的纖維素包裝袋相比,打開密封的特衛強包裝袋產生的顆粒(尺寸為 0.5 微米和 5 微米)數量不到其十分之一。顆粒污染風險的可能性評分為 1。嚴重程度評分為 3,因為這可能會影響產品質量,盡管不像導致產品被拒收那樣嚴重。通過顆粒監測,可檢測性評分為 3,因此風險優先數(RPN)為 9,處于綠色區域。
If cellulose material is used, the probability of particle contamination is considered as a rating of 3. This would lead to an RPN=27, which falls in the yellow area, corresponding to a moderate risk according to Table 4.
如果使用纖維素材料,顆粒污染的可能性評分為 3。這將導致風險優先數(RPN)為 27,處于黃色區域,根據表 4,對應中等風險。

Figure 6 Risk evaluation of the steps of preparation of the stopper bowl for sterilization and the sterilization process
圖 6 膠塞滅菌準備步驟以及滅菌過程的風險評估
Sterilization of the Stopper Bowl in Autoclave
膠塞在高壓滅菌器中的滅菌
A validated steam sterilization process with a well-maintained autoclave carries a low risk, as it is considered that a Bowie-Dick Test is used every day before using the autoclave. The risk in this step is also tightly associated with the wrapping material used. If the material considered is Tyvek, and there is data from the supplier on—
在維護良好的高壓滅菌器中進行經過驗證的蒸汽滅菌過程,其風險較低,因為通常認為在使用高壓滅菌器之前每天都會進行鮑伊 - 狄克測試(Bowie-Dick Test)。這一步驟中的風險也與所使用的包裝材料緊密相關。如果所考慮的材料是特衛強(Tyvek),并且供應商提供以下數據:
Good steam penetration
良好的蒸汽穿透性
Reproducibility during the wrapping process to ensure the same barrier to steam every time
包裝過程中的可重復性,以確保每次對蒸汽具有相同的阻隔性
Good microbial barrier
良好的微生物阻隔性
Low particle generation
產生較少的顆粒
—then it is considered that the wrapping has a view window to allow the visual inspection of the parts for the rest of humidity after the cycle. The risk can be assessed as very low, giving an RPN=5.
那么可以認為這種包裝有一個觀察窗,以便在滅菌循環后目視檢查部件是否殘留水分。可認為該步驟風險非常低,風險優先數(RPN)為 5。
Transfer of the Sterilized Stopper Bowl from Grade D to Grade C
已滅菌膠塞從 D 級區域轉移至 C 級區域
The transfer of materials into and out of the cleanrooms and critical zones is one of the greatest potential sources of contamination (2). The key to minimizing microbial and particulate contamination is to assess the possibilities available at the facility and look into the most practical and appropriate way to mitigate those risks. Different ways to perform material transfer include:
物料進出潔凈室和關鍵區域的轉移是最大的潛在污染源之一(參考文獻 2)。將微生物和顆粒污染降至最低的關鍵在于評估工廠現有的可能性,并尋找最實際且合適的方法來降低這些風險。進行物料轉移的不同方式包括:
Sterilization (double-door/ended) or depyrogenation tunnel
滅菌(雙門)或除熱原隧道
VHP or biodecontamination method
汽化過氧化氫(VHP)或生物凈化方法
Removing outer layer of sterilized packaging
移除已滅菌包裝的外層
Manual disinfection
手動消毒
The most practical way to minimize contamination risk is to use a double-door autoclave (2), but this is not always possible.
將污染風險降至最低的最實際方法是使用雙門高壓滅菌器(參考文獻 2),但并非總是能夠做到這一點。
The recommended sterilization wrapping for the stopper bowl when there is no double-door autoclave is a cover plus two sterilization-wrapping bags of flexible Tyvek. In the example in Figure 2 (B), the transfer of the stopper bowl will be done by removing the outer layer of sterilization wrapping. This method is preferred over manual disinfection due to the following:
在沒有雙門高壓滅菌器的情況下,推薦用于膠塞的滅菌包裝是一個外罩加上兩個由柔性特衛強(Tyvek)制成的滅菌包裝袋。在圖 2(B)的示例中,膠塞的轉移將通過移除滅菌包裝的外層來完成。由于以下原因,這種方法優于手動消毒:
It eliminates a manual procedure with all its inherent challenges in reproducibility.
它消除了手動操作及其在可重復性方面的固有挑戰。
The manual disinfection in this case is especially challenging since the outer sterilization wrapping layer is flexible and, therefore, difficult to cover with a wipe.
在這種情況下,手動消毒尤其具有挑戰性,因為外層滅菌包裝是柔性的,因此很難用擦拭布覆蓋。
Removing an outer sterilization layer is faster than wiping with disinfectant, and it bypasses wet-contact times.
移除外層滅菌包裝比用消毒劑擦拭更快,并且可以避免濕接觸時間。
The recommended procedure for the transfer of the stopper bowl from Grade D to Grade C is:
將膠塞從 D 級區域轉移至 C 級區域的推薦程序如下:
The cart is removed from the autoclave, and the parts are inspected for any condensation.
將推車從高壓滅菌器中取出,并檢查部件是否有任何冷凝現象。
The stopper bowl is placed on the cart side Grade D and driven into the material airlock.
將膠塞放置在 D 級區域的推車上,然后將推車駛入物料氣閘。
With the help of another operator, and under continuous laminar airflow, the outer layer of wrapping is removed, and the stopper bowl is placed on the cart side Grade C.
在另一名操作人員的幫助下,在持續層流氣流下,移除包裝的外層,然后將膠塞放置在 C 級區域的推車上。
The stopper bowl remains with an inner layer of wrapping and can be stored or be directly installed in the filling line.
膠塞保留內層包裝,可以儲存或直接安裝在灌裝線上。
Without a continuous particle-monitoring in the material airlock, the step is ranked as moderate to high risk with an RPN=45. It is therefore recommended, if possible, to avoid having to do a manual material transfer and to automate the process with a decontamination chamber or a double-door autoclave.
由于在物料氣閘中沒有持續的顆粒監測,這一步驟被評為中高風險,風險優先數(RPN)為 45。因此,建議如果可能的話,避免進行手動物料轉移,并通過凈化室或雙門高壓滅菌器使該過程自動化。
Unwrapping and Installation of the Stopper Bowl in the Filling Line
膠塞在灌裝線上的拆包和安裝
This step poses a particular high risk when dealing with isolators, as the surrounding environment is a Grade C environment, for which the operators have a lower degree of gowning in comparison to a Restricted Access Barrier System (RABS), where the surrounding environment is Grade B. For this reason, to mitigate the risks during the unwrapping and installation of the stopper bowl, the operators should wear extra gowning such as face masks, goggles and Tyvek sleeves. On the other hand, isolators provide an “extra layer of protection” due to the biodecontamination with VHP, performed right before production starts. This helps mitigate any risk of contamination that could happen during the transfer of the stopper bowl and the unwrapping and installation process. In recent years, RABS has also employed automated VHP biodecontamination before production starts by applying VHP in the Grade B rooms and Grade A RABS at the same time. The recommended procedure would be:
在處理隔離器時,這一步驟具有特別高的風險,因為周圍環境是 C 級環境,與限制進入屏障系統(RABS)相比,操作人員在 C 級環境中的著裝要求較低,而在 RABS 中,周圍環境是 B 級。因此,為了降低膠塞拆包和安裝過程中的風險,操作人員應穿戴額外的防護裝備,如口罩、護目鏡和特衛強(Tyvek)袖套。另一方面,由于在生產開始前立即進行汽化過氧化氫(VHP)生物凈化,隔離器提供了 “額外的防護層”。這有助于降低在膠塞轉移以及拆包和安裝過程中可能發生的任何污染風險。近年來,限制進入屏障系統(RABS)也在生產開始前通過同時在 B 級房間和 A 級 RABS 中應用汽化過氧化氫(VHP)來進行自動化的生物凈化。推薦程序如下:
The wrapped stopper bowl should be transported with the cart until right before the limit between Grade C and Grade A areas (punctuation line on Figure 2). The unwrapping should happen inside the area between the punctuation line and the isolator doors. In this area, continuous laminar flow should be coming from the isolator Grade A area.
已包裝的膠塞應隨推車運輸,直到 C 級和 A 級區域之間的界限處(圖 2 中的標點線)。拆包應在標點線和隔離器門之間的區域內進行。在該區域,應從 A 級隔離器區域有持續的層流氣流。
Continuously under the laminar flow, with the help of an operator, the outer cover of the stopper bowl should be unwrapped.
在持續的層流氣流下,在一名操作人員的幫助下,應拆除膠塞的外罩。
Both operators should place the stopper bowl on the line for installation.
兩名操作人員應將膠塞放置在生產線上進行安裝。
The last cover can be left on the stopper bowl until after the VHP cycle and right before aseptic production starts.
膠塞的最后一層包裝可以保留,直到汽化過氧化氫(VHP)循環結束且無菌生產開始前。
This transfer step is the most critical in this scenario with an RPN=30. The high number is due to a lack of continuous real-time EM that could detect any issues during the procedure. This requires relying on particle monitoring and aseptic process simulation to ensure the procedure is functioning as expected.
在這種情況下,這一轉移步驟是最關鍵的,風險優先數(RPN)為 30。數值較高是因為缺乏能夠在操作過程中檢測到任何問題的持續實時環境監測(EM)。這需要依靠顆粒監測和無菌工藝模擬,以確保該操作按預期進行。
As defined in this example, an RPN=30 demands measures to be in place to control the risk such as:
正如本示例中所定義的,風險優先數(RPN)為 30 需要采取適當的措施來控制風險,例如:
Supervision by other operators
由其他操作人員進行監督
Good aseptic technique
良好的無菌技術
Well-written procedures
編寫完善的操作程序
This should help in mitigating the risk in the transfer and installation step.
這將有助于降低轉移和安裝步驟中的風險。

Figure 7 Risk evaluation of the unwrapping and installation of the stopper bowl in the filling line圖 7 膠塞斗在灌裝線上拆包和安裝的風險評估
Scenario 2: Stopper Bowl is Nondetachable
場景 2:膠塞不可拆卸
In this case, a manual cleaning and disinfection of the stopper bowl can be used as an alternative procedure. A typical lifecycle of the stopper bowl for this scenario can be seen in Figure 8.
在這種情況下,可采用對膠塞進行手動清潔和消毒的替代程序。這種場景下膠塞的典型生命周期如圖 8 所示。

Figure 8 Lifecycle of a Nondetachable Stopper Bowl
圖 8 不可拆卸膠塞斗的生命周期
Transfer and Preparation of Cleaning, Disinfection and Rinsing Agents
清潔、消毒劑的轉移及準備
The cleaning and disinfecting agents used inside Grade A and Grade B areas should be sterile prior to use (2).
在 A 級和 B 級區域內使用的清潔和消毒劑在使用前應是無菌的(參考文獻 2)。
The transfer of these materials will depend on the format in which they are packed. If the products used are ready-to-use (RTU) wipes, they come sterile and double-bagged. These can be transferred using the material airlock shown in Figure 2 by removing the first packaging layer inside the Grade D area and placing the wipes on the cart side of the Grade C area. The last packaging layer will be removed inside the isolator or in the material airlock of the isolator, prior to use of the wipes.
這些物料的轉移取決于它們的包裝形式。如果使用的產品是即用型(RTU)擦拭布,它們是無菌的且采用雙層包裝。可以使用圖 2 所示的物料氣閘進行轉移,在 D 級區域內移除第一層包裝,然后將擦拭布放置在 C 級區域的推車上。在使用擦拭布之前,應在隔離器內或隔離器的物料氣閘中移除最后一層包裝。
In case the product comes in spray form and is double-packed, the same procedure for material transfer can be performed. The spray and trigger may come separately; both are to be transferred in the same way. Right before starting the procedure, the trigger can be pushed into the spray bottle inside the material airlock of the isolator and then transferred inside the isolator.
如果產品是以噴霧形式且采用雙層包裝,則可以執行相同的物料轉移程序。噴霧瓶和噴頭可能是分開的,兩者都應以相同的方式進行轉移。在開始操作之前,應在隔離器的物料氣閘內將噴頭安裝到噴霧瓶上,然后轉移到隔離器內。
Sterile polyester wipes can be transferred either by removing the outer packaging layer or by disinfecting the packaging every time it is transferred to a higher classification. If the disinfection method is used, it should be performed in the lower classification area, while the wet-contact times should be observed in the higher classification area of the airlocks.
無菌聚酯擦拭布可以通過移除外包裝層或在每次轉移到更高等級區域時對包裝進行消毒的方式進行轉移。如果采用消毒方法,則應在較低等級區域內進行,同時應在氣閘的較高等級區域內遵守濕接觸時間的要求。
The risks associated with the preparation of the cleaning and disinfection (C&D) agents will increase if the use-dilution of detergent/disinfectant needs to be prepared and filtered under a laminar flow inside the Grade A area. The more manipulation needed for the preparation, the higher the risks for contamination and compromise of the sterile state of the agent used.
如果需要在 A 級區域內的層流條件下對洗滌劑 / 消毒劑進行使用稀釋和過濾,那么與清潔和消毒劑(C&D)準備相關的風險將會增加。準備過程中需要的操作越多,所使用的試劑被污染以及破壞其無菌狀態的風險就越高。
To evaluate the risks of this step, the following need to be considered:
為了評估這一步驟的風險,需要考慮以下因素:
Use of RTU wipes – low risk on preparation; otherwise, high risk
使用即用型(RTU)擦拭布 —— 準備過程中風險較低;否則,風險較高
Manual or automated material transfer (e.g., with a decontamination chamber)
手動物料轉移或自動化物料轉移(例如,使用凈化室)
Controls in place during the material transfer, such as continuous particle-monitoring in the material airlock
物料轉移過程中的控制措施,例如在物料氣閘中進行持續的顆粒監測
For this example, the material transfer will be considered to be manual and RTU wipes are used. The probability of a risk happening during a manual material transfer is considered as having a rating of 3. Since there is usually no continuous particle monitoring or EM in place in a material airlock, it is difficult to detect if something goes wrong during the process. The only control in place is visual inspection. Detectability is assigned a rating of 5. The severity is classified as 2 because there is a VHP step prior to production to eliminate potentially introduced contamination. This step is identified as middle-to-high risk (RPN=30). If a decontamination chamber is available, the probability would decrease to 1, and the step would have an RPN of 10.
在本示例中,物料轉移被認為是手動的且使用即用型(RTU)擦拭布。手動物料轉移過程中發生風險的可能性評分為 3。由于在物料氣閘中通常沒有持續的顆粒監測或環境監測(EM),因此很難檢測到過程中是否出現問題。現有的唯一控制措施是目視檢查。可檢測性評分為 5。嚴重程度分類為 2,因為在生產前有汽化過氧化氫(VHP)步驟來消除潛在引入的污染。這一步驟被確定為中高風險(風險優先數(RPN)=30)。如果有凈化室,可能性將降至 1,該步驟的風險優先數(RPN)為 10。
Manual Cleaning of the Stopper Bowl
膠塞的手動清潔
It is important to understand the possible residues that need to be cleaned to select the right cleaning agent. Hard-to-clean residues such as silicone oil usually need an alkaline-formulated detergent. Other easier-to-clean residues might be cleaned using a neutral-formulated detergent, H2O2, 70% Isopropyl alcohol (IPA) or Water for injection (WFI). When cleaning with a formulated chemistry, WFI or 70% IPA applied in wipes can be used to remove surfactant residue.
了解需要清潔的可能殘留物以選擇合適的清潔劑是很重要的。像硅油這樣難以清潔的殘留物通常需要堿性配方的洗滌劑。其他較易清潔的殘留物可以使用中性配方的洗滌劑、過氧化氫、70% 異丙醇(IPA)或注射用水(WFI)進行清潔。當使用配方化學制劑進行清潔時,可以使用沾有注射用水或 70% 異丙醇的擦拭布來去除表面活性劑殘留物。
The manual cleaning of the stopper bowl will be done with the doors open, unless it is possible using another method. Besides the inherent risks of the manual cleaning itself, other measures are necessary to minimize the risk of particle and microbial contamination during the procedure, such as:
膠塞的手動清潔通常需要在門打開的情況下進行,除非可以使用其他方法。除了手動清潔本身固有的風險之外,還需要采取其他措施來將操作過程中顆粒和微生物污染的風險降至最低,例如:
Laminar flow working throughout the process
在整個過程中保持層流
Extra gowning, for example, face masks, goggles, and Tyvek sleeves
穿戴額外的防護裝備,例如口罩、護目鏡和特衛強(Tyvek)袖套
Practice of first cleaning nonproduct-contact areas or low-risk areas, followed by high-risk areas like the stopper bowl in the cleaning procedure.在清潔程序中,先清潔非產品接觸區域或低風險區域,然后清潔像膠塞斗這樣的高風險區域
As a critical indirect-surface area, the cleaning of the stopper bowl needs to be validated using visual inspection and both analytical and sampling methods. Analytical methods will depend on the residue to be traced. Total organic carbon (TOC) analysis can be challenging due to interference coming from IPAs commonly used in these areas. Therefore, HPLC and conductivity methods are preferred. Another challenge is the calculation of a cleaning limit for a stopper bowl where there is no direct contact with the product, but contact with a primary-packaging material.
作為關鍵的間接表面區域,膠塞的清潔需要通過目視檢查以及分析和取樣方法來進行驗證。分析方法將取決于要追蹤的殘留物。由于這些區域通常使用異丙醇會產生干擾,因此總有機碳(TOC)分析可能具有挑戰性。因此,更傾向于使用高效液相色譜(HPLC)和電導率方法。另一個挑戰是計算與產品無直接接觸但與初級包裝材料接觸的膠塞斗的清潔限度。
Manual cleaning introduces the usual risks associated with reproducibility and is more carefully reviewed by authorities. Measures that can be taken to minimize these risks are:
手動清潔會帶來與可重復性相關的常見風險,并且會受到監管部門更嚴格的審查。可以采取以下措施來將這些風險降至最低:
Procedures should be easy to follow
操作程序應易于遵循
Standard operating procedures should be detailed, and operators should be trained frequently
標準操作程序應詳細,并且操作人員應經常接受培訓
Procedures should be supervised at a certain frequency or every time (four-eyes principle)
應按一定頻率或每次都對操作程序進行監督(雙人復核原則)
Monitoring procedures, like swabbing, to confirm the cleaning process was effective should be done after each cleaning or at least with a higher frequency as done in automated cleaning processes (6).
應進行監測程序,例如擦拭取樣,以確認清潔過程是有效的,每次清潔后都應進行,或者至少像在自動化清潔過程中那樣以更高的頻率進行(參考文獻 6)。
Some of these measures might be challenging to execute, depending on the production schedule, such as frequent training, observations of the process and periodic swabbing of the surface. Nonetheless, a compromise should be found in which the risks can be minimized, and resources are not exceeded.
根據生產計劃,其中一些措施可能執行起來頗具挑戰性,比如頻繁的培訓、對生產過程的觀察以及定期的表面擦拭取樣。盡管如此,還是應該找到一種折中的辦法,既能將風險降至最低,又不會過度消耗資源。
As mentioned, RTU wipes not only simplify the material transfer but also eliminate the need to prepare the use-dilution inside the Grade A area, thereby minimizing risks and the number of manual activities overall. The use of laboratory coupon studies can help explore and define the right procedure such as how many wipes to use, how many strokes per wipe are needed and which kind of wiping pattern is optimal (e.g., unidirectional overlapping strokes, back and forth). Rinsing with 70% IPA to remove the excess of surfactants can also be simulated in the laboratory.
如前所述,即用型(RTU)擦拭布不僅簡化了物料轉移,還消除了在 A 級區域內準備使用稀釋液的需求,從而總體上降低了風險并減少了手動操作的數量。使用實驗室試片研究可以幫助探索和確定正確的操作程序,例如使用多少擦拭布、每次擦拭需要多少次擦拭以及哪種擦拭模式是最佳的(例如,單向重疊擦拭、來回擦拭)。還可以在實驗室中模擬使用 70% 異丙醇進行沖洗以去除多余的表面活性劑。
The main risk during this step is not having a successful cleaning. There is a risk of introducing contamination during this intervention; however, manufacturers with experience in this type of cleaning report that the risk is very low. The probability of this risk should be revised and assessed based on historical data and the experience of each manufacturer as this will depend on the residue, the cleaning agents used, the procedure and the design of the filling line. For this example, the residue is considered to be silicone oil. The probability of an unsuccessful cleaning can be considered a rating of 3or even a5because this is a manual process, and silicone oil is a hard-to-clean residue. Due to the difficulties of assessing silicone residue via visual inspection, the detectability is assigned as a rating of5. Severity is assigned a2rating as this residue would not impact product quality. This gives an RPN between30and50, middle-to-high risk. The probability of this risk can be reduced to rating of3if a laboratory model is used to design the cleaning procedure. This would result in an RPN between10and30. If the residue is easy to clean, the probability of having an unsuccessful cleaning can be reduced to a 1, resulting in an RPN of10.
這一步驟的主要風險是清潔不成功。在這一干預過程中存在引入污染的風險;然而,在這種類型的清潔方面有經驗的制造商報告說,這種風險非常低。應根據歷史數據和每個制造商的經驗來修訂和評估這種風險的可能性,因為這將取決于殘留物、所使用的清潔劑、操作程序和灌裝線的設計。在本示例中,殘留物被認為是硅油。清潔不成功的可能性可被認為評分為 3 甚至 5,因為這是一個手動過程,并且硅油是一種難以清潔的殘留物。由于難以通過目視檢查評估硅酮殘留物,可檢測性評分為 5。嚴重程度評分為 2,因為這種殘留物不會影響產品質量。這使得風險優先數(RPN)在 30 到 50 之間,屬于中高風險。如果使用實驗室模型來設計清潔程序,這種風險的可能性可以降低到評分為 3。這將導致風險優先數(RPN)在 10 到 30 之間。如果殘留物易于清潔,清潔不成功的可能性可以降低到評分為 1,風險優先數(RPN)為 10。

Figure 9 Risk evaluation of the transfer of cleaning and rinsing agents and the manual cleaning step of a nondetachable stopper bowl
圖 9 不可拆卸膠塞的清潔和沖洗劑轉移以及手動清潔步驟的風險評估
Manual Disinfection of Stopper Bowl
膠塞的手動消毒
A sporicidal agent is recommended to be used as disinfectant as this is an indirect surface near the final filling of the product. A sporicidal agent, such as a peracetic acid/H2O2 blend, will ensure full microbial inactivation. IPA 70% applied in wipes is recommended as the rinsing method to allow for evaporation after the rinse step is completed.
建議使用殺孢子劑作為消毒劑,因為膠塞是靠近產品最終灌裝處的間接表面。像過氧乙酸 / 過氧化氫混合物這樣的殺孢子劑能確保完全滅活微生物。建議使用沾有 70% 異丙醇的擦拭布作為沖洗方法,以便在沖洗步驟完成后使其蒸發。
As the surface of the stopper bowl is in direct contact with stoppers (primary product-contact surfaces), the stopper bowl requires cleaning validation. This means that that the rinsing step should be demonstrated as able to remove the sporicidal agent below a calculated cleaning limit. The sporicidal agent should have a Permitted Daily Exposure (PDE) or Acceptable Daily Exposure (ADE) value available, and the shared surface area should be considered for the calculation of the cleaning limit.
由于膠塞的表面與膠塞(主要的產品接觸表面)直接接觸,膠塞需要進行清潔驗證。這意味著沖洗步驟應證明能夠將殺孢子劑去除到低于計算出的清潔限度。殺孢子劑應具有允許的每日暴露量(PDE)或可接受的每日暴露量(ADE)值,并且在計算清潔限度時應考慮共享的表面積。
The risks associated with this step include:
與這一步驟相關的風險包括:
Manual disinfection application and being able to cover the surfaces of the stopper bowl
手動進行消毒操作并確保能夠覆蓋膠塞的表面
Unidirectional overlapping strokes are challenging to perform due to irregularities on the stopper bowl surface
由于膠塞表面不平整,進行單向重疊擦拭操作具有挑戰性
Longer wet-contact times in a Grade A area—The effectiveness of the disinfection is defined as a certain wet-contact time needed to achieve a certain 10-log reduction (7). In a Grade A area, due to the high number of air exchanges, it is challenging to reach wet-contact times longer than five minutes. For Grade A/ISO 5 areas, however, the acceptable microbial count should be 0 (no growth) (2). According to USP 43, Chapter <1116>, samples with contamination should not exceed 0.1% of the total samples collected. In other words, out of 1,000 samples, 999 should show no growth. This implies that, for example, a 10-minute contact time with a sporicidal agent to achieve a 6-log reduction might not be required. A shorter wet-contact time, based on achievable values, can be validated for a Grade A area, reducing the risk of the contact time not being respected. The effectiveness of the procedure can be confirmed during aseptic process simulation and in disinfectant in-situ testing. (See Figure 10.)
在 A 級區域內較長的濕接觸時間 —— 消毒的有效性定義為達到一定的對數減少所需的特定濕接觸時間(參考文獻 7)。在 A 級區域,由于換氣次數較多,要達到超過五分鐘的濕接觸時間具有挑戰性。然而,對于 A 級 / ISO 5 區域,可接受的微生物計數應為 0(無生長)(參考文獻 2)。根據美國藥典(USP)第 43 版第 <1116> 章,受污染的樣品不應超過所采集總樣品的 0.1%。換句話說,在 1000 個樣品中,999 個應無微生物生長。這意味著,例如,為達到 6 個對數減少而使用殺孢子劑接觸 10 分鐘可能并非必要。對于 A 級區域,可以根據可實現的值驗證較短的濕接觸時間,從而降低不遵守接觸時間的風險。該程序的有效性可以在無菌工藝模擬和消毒劑原位測試中得到確認。(見圖 10)

Figure 10 Risk evaluation of the manual disinfection step of a nondetachable stopper bowl
圖 10 不可拆卸膠塞手動消毒步驟的風險評估
Conclusion
結論
The risk-assessment exercise for both scenarios, involving detachable and nondetachable stopper bowls, aimed to identify the major challenges in each case and determine the best recommendations or control measures to mitigate contamination risks.
針對可拆卸和不可拆卸膠塞這兩種情況所進行的風險評估工作,旨在識別每種情況下的主要挑戰,并確定最佳的建議或控制措施,以降低污染風險。
It is challenging to reach a conclusion about which scenario poses the lowest risks due to the numerous assumptions made during the analysis. Each manufacturer should arrive at their own conclusions based on their procedures and controls in place. An overview of the advantages, disadvantages and recommendations for both scenarios are given in Table 5.
由于在分析過程中做出了大量假設,要得出哪種情況風險最低的結論具有一定挑戰性。每個制造商都應根據自身的操作程序和已實施的控制措施得出各自的結論。表 5 給出了這兩種情況的優缺點以及建議的概述。
Table 5 Summary of advantages, disadvantages and recommendations of both scenarios
表 5 兩種情況的優缺點及建議總結
The simplest way to comply with EU GMP Annex 1 is to use a detachable stopper bowl, and new filling lines will meet this requirement. Alternatively, redesigning the production line for nondetachable parts is an option, though it may be prohibitively expensive for some companies. Additionally, the more indirect surfaces that cannot be sterilized, the greater the effort and cost required to redesign the filling line. In such cases, a risk-management approach can justify an alternative procedure, such as manual C&D.
符合歐盟藥品生產質量管理規范(GMP)附件 1 的最簡單方法是使用可拆卸的膠塞斗,并且新的灌裝線應滿足這一要求。或者,對不可拆卸部件的生產線進行重新設計也是一種選擇,不過對于某些公司來說,這可能成本過高。此外,無法進行滅菌處理的間接表面越多,重新設計灌裝線所需的精力和成本就越大。在這種情況下,采用風險管理方法可以為諸如手動清潔和消毒(C&D)之類的替代程序提供合理依據。

來源:Internet


